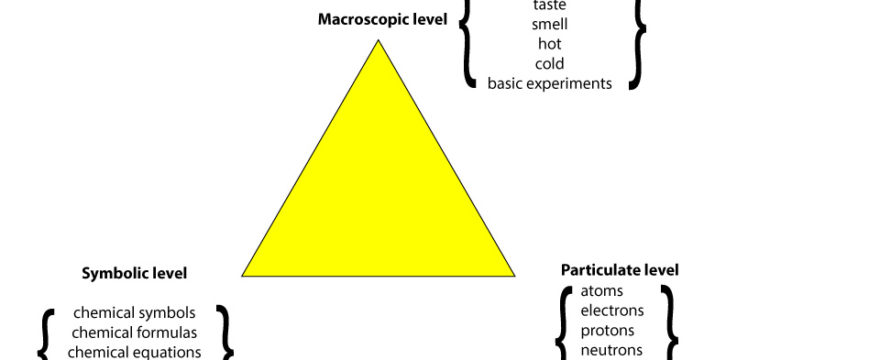
What’re the Three Levels of Representation in Chemistry?
The three levels of representation are:
- Macroscopic
- Particulate
- Symbolic
What’s the Macroscopic level of representation?
This level describes the things we can perceive with our senses (smell, taste, sight, touch, and hear) or measure with basic instruments such as thermometer. Some examples of properties we can determine at the macroscopic level include color, taste, texture, temperature, pressure, or density. For example, you can see and describe the color of things you observe. You can also describe the taste of things you taste and texture of things you feel. These properties are usually produced as a result of the collective interactions of atoms or molecules at the particulate or molecular level of the substance. What then is the particulate level?
What’s the Particulate level of representation?
This level describes the things we cannot perceive with our senses, but they are there. These things include atoms, molecules, ions, electrons, protons, and neutrons. Because these particles are microscopic and invisible to our eyes, the particulate level is sometimes called the, atomic, submicroscopic, microscopic or molecular level. The way these particles arrange and interact at this level determine the properties we observe at the macroscopic level. Because of this, the particulate level is the most valuable level to chemists, and so chemists usually rely on technology to extend their senses so that they can study and understand the behavior of molecules at the molecular level. Once chemists understand their behavior, they can take advantage of this understanding to design materials with unique properties. From this, we can see that science and technology depend on each other. That is technology is necessary to advance science and science is necessary to advance technology.
What’s the Symbolic level of representation?
This level uses symbols to describe the invisible particles that exist at the particulate level and their relationship to one another. These symbols include
- Chemical symbols
For example, the chemical symbol for sodium is Na
- Chemical formulas
For example, the chemical formula for vinegar is CH3COOH
- Mathematical formulas
For example, the density (D) of substance is a ratio of its mass (M) to its volume (V). We can express this relationship as: D = M/V
- Chemical equations
For example, hydrogen reacts with oxygen to form water. We can write a balanced chemical equation for this reaction as: 2H2 + O2 → 2H2O
- Models
For example, chemists usually draw a circle to depict an atom. Keep in mind that a model is not the actual thing is representing. It is an approximation to help us study and predict the properties and behavior of the actual thing. In science, models can be abstract, conceptual, mathematical, and graphical.
What’s the Triplet Representation?
When we write each level on one of the vertices of a triangle, we get what we call the Triplet Representation. The triplet representation was proposed by a well known chemical educator called Alex Johnstone.
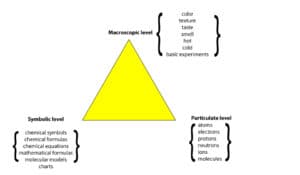
How can the Triplet Representation Help Us Understand Chemistry?
It can help us in many ways. First, it hints at the idea that when we study a chemical substance or phenomena, we should at least think about it at the macroscopic-, particulate-, and symbolic- level.
What does that mean?
Let’s explain this further by using a typical example we have all experienced in our lives: water.
At the macroscopic level, water can exists as a solid, liquid or gas. When it exists as pure liquid it is colorless. When it exists as solid like ice or snow it is white. When it exists as a gas, it is invisible.
At the particulate level, water consists of two hydrogen atoms united (bonded) with one oxygen atom. Thus, two parts of hydrogen molecules always react with one part of oxygen molecules to make 2 parts of water molecules. Depending on energy, temperature, and attractive forces, water molecules can arrange at the molecular level to form solid, liquid or gas.
At the symbolic level, water is depicted as H2O, where H: hydrogen, and O: oxygen. The subscripts reflect the ratio in which these two different atoms always react to form water. Thus, you always need 2 parts of hydrogen molecules to 1 part of oxygen molecules. As you can tell, the subscript “1” is not written by oxygen. When the subscript of any atom is “1”, it is usually left out when we write its chemical formula.
What is Conceptual Understanding?
Conceptual understanding is achieved when a student can explain beyond the macroscopic level the cause of a chemical phenomenon, just like we did with water.
Why is chemistry difficult to understand?
As you can tell, we can’t perceive the particulate level with our senses. This difficiency makes the particulate level abstract and difficult to understand. However, with the help of technology chemists can extend their senses so that they can explore the behavior and structure of molecules at the molecular level. Once chemists understand the structure and behavior of molecules, they can often predict and make new materials with unique properties.
How can we apply the three-levels to teach a chemistry concept?
At the:
- Macroscopic level: provide students with hands-on activities or experiments so that they can explore to discover things for themselves. For example, if you want to teach about density, let students drop different objects of the same mass or volume in water to see whether an object will float or sink. Once they do that, let them determine the mass and volume of the objects they used.
- Particulate level: let students draw particle models to explain why they think two objects can have the same mass, but one object may float while the other may sink. And why two objects can have the same volume, but one may float while the other may sink in water.
- Symbolic level: let students use symbols to discuss the relationship between mass and volume for objects that float and those that sink based on the data they gathered during the activities.
This same approach can be applied to teach chemical reactions and many concepts in chemistry. But in some situations you may need to show animations of the molecular process to help students visualize how the molecules are behaving at the molecular level.
As you do this in your class, you may need to guide students through discussion and questioning so that they can connect all the three levels to the chemistry concept they are learning about.
Check your understanding
- Obtain any solid object and describe it according to the:
- Macroscopic level
- Particulate level
- Symbolic level