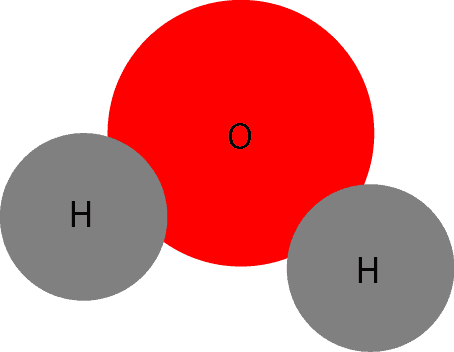
What’s a Compound?
A compound is formed when at least two different atoms react in specific amounts (fixed-mass ratios) to make a new substance. Pay attention to the text in bold: different atoms. Let’s use the model below to illustrate the concept.
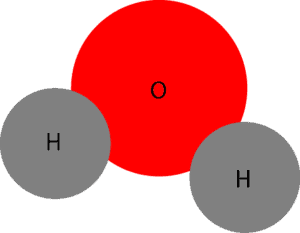
As you can tell, the red circle depicts oxygen (O) atom, while the grey circle depicts hydrogen atom. When these atoms chemically react, they form a compound called water: H2O. When several of these water particles are attracted to each other, they form a free flowing liquid we can see, feel, fetch and do different things with it. The model below shows several water particles attracted to each other.
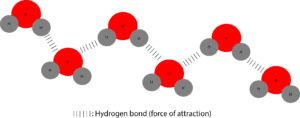
The dashes you see are showing the force of attraction between water molecules. Here the attraction is between an oxygen atom on one end of a water molecule with a hydrogen atom on the other end of another water molecule. This type of weak attraction is called a hydrogen bond.
Notice that we have used the words particles and molecules interchangeably. Why did we do that? For nature to make a substance we can see and feel, she must put several of these water particles together. And for each small particle, chemists call it a molecule. In other words, a compound such as water that can exists as a solid, liquid, or gas usually consists of several molecules of water put together. So we can say that the smallest particle of a compound that can exist is always a molecule. Which is why scientists often like saying this or that substance consist of this or that type of molecule.
Notice that the term molecule can also be applied to elements. To read more about elements that can exist as molecules, click Here.