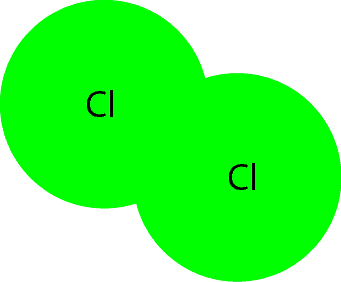
What is a Molecule?
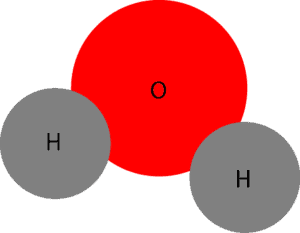
A molecule is formed when two or more atoms of the same or different type unite to form a bond (a force of attraction between atoms). Notice the text in bold: atoms of same or different type. Atoms of the same or different types means that to form a molecule nature or chemists can use atoms of the same element or atoms of different elements. Here is a model that illustrates a molecule of water. As you can see it consists of atoms of different elements (hydrogen and oxygen).
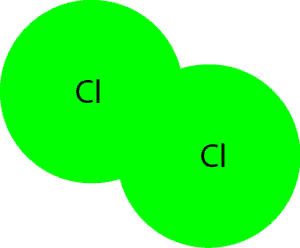
Here is another model that illustrates a molecule of chlorine that consists of atoms of chlorine (atoms of same type (element)).
A molecule is also the smallest particle of a chemical compound that can take part in a chemical reaction. To read more about a compound, click here.
Note! To show bonding between atoms, we usually construct the circles to intersect. The area of intersection is the bonded region.
Check your understanding
- Can a compound also exist as a molecule? Explain