What’s an Element?
An element consists of atoms of the same type. Now, pay attention to the words in bold: atoms of same type. What does that mean? Let’s say you have in your hand an Aluminum foil, this solid aluminum foil in your hand is made of several atoms of aluminum packed together tightly to form the solid aluminum foil. Now let’s try to use the model below to illustrate the concept.
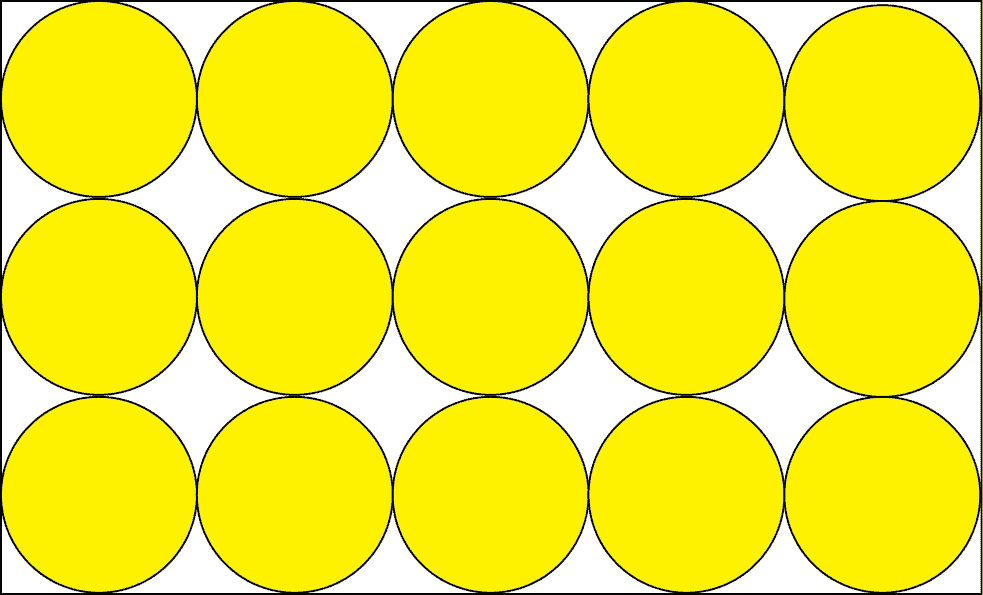
As you can see, the whole rectangular block is your solid aluminum metal. The individual yellow circles are the aluminum atoms. Now how can you tell from the drawing whether the element is a solid, liquid, or gas? You can tell by looking at how closely packed the atoms are to one another. Since the atoms are closely packed, the drawing depicts a solid.
Do some Elements exist as Molecules?
Yes! There are a few elements that consists of molecules. Most of these elements exist in nature as gases. Among them are hydrogen, oxygen, nitrogen, fluorine, chlorine, iodine,and bromine. What is a molecule? A molecule is formed when two or more atoms of the same or different type unite to form a bond (a strong attraction between atoms). Here is a model that illustrates a collection of oxygen molecules.
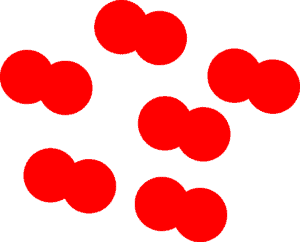
As you can see, the red circles are intersecting, this intersection is what we use to illustrate that the two atoms are bonded to form a molecule. Again how can you tell whether the drawing depicts a solid, liquid, or gas? You can tell by looking at how closely packed the molecules are. Here the molecules are reasonably spaced, depicting a gas.