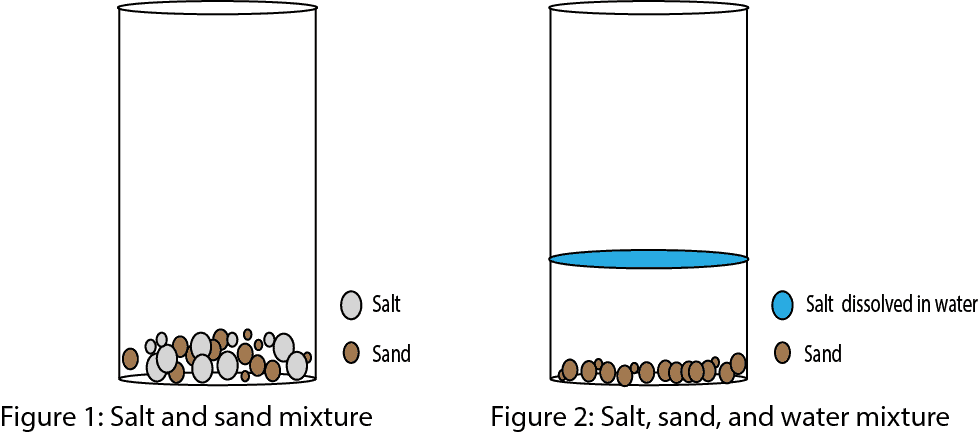
What’s a Mixture?
A mixture is when two or more chemicals are intermingled (mixed) with each other. Pay attention to the text in bold: intermingled. When you add salt to sand, you have created a mixture in which the two chemicals are intermingled with each other. The two chemicals are intermingled in the sense that the two chemicals haven’t really reacted with each other to create a new substance. In other words, the salt still remains as salt and the sand as sand in the mixture.
Now, assume you add water, will the mixture still remain a mixture? Yes, it will still remain a mixture, but the salt will now be dissolved in the water, while the sand won’t. You will now have a mixture of salt, sand, and water, but with the sand settled at the bottom of the container. As you can tell, the composition (makeup) of the mixture is not the same everywhere in the mixture. Thus, there will be more sand at the bottom and more water on top of the sand in the mixture. So if you sample from the mixture you can’t guarantee your sample will contain equal amounts of the chemicals in the mixture. So you see, one property of a mixture is that its composition (makeup) is not fixed. Let’s use the models below to illustrate the concept.
How’re Mixtures Classified?
Mixtures can be classified into two groups: homogeneous and heterogeneous mixture.
What’s a Homogeneous mixture?
A homogeneous mixture (also called a solution) is when the chemicals in the mixture are evenly distributed throughout the mixture. Let’s use the model below to illustrate the concept.
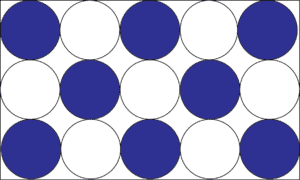
Say you dissolved sugar in water to make a sugar solution (homogeneous mixture). After making your solution, you sampled it from the top, middle, and bottom and noticed that regardless of where you sampled, the solution still tasted sweet. From your observations, you then drew a model like the one above, where the white circles depict sugar molecules and the blue circles water molecules. From the model, you can see that the molecules of water and sugar are evenly distributed in the entire solution. Thus, every sugar molecule is completely surrounded by water molecules in the mixture. But keep in mind that uniform distribution does not mean fixed composition.
What’s a Heterogeneous mixture?
A heterogeneous mixture is formed when the chemicals in the mixture are not uniformly distributed throughout the mixture. For instance, if you add sand to salt, no amount of grinding can make sand and salt form a homogeneous mixture like salt and water. Thus, we can say that a mixture of sand and salt will form a heterogeneous mixture. Here is a model to illustrate the concept.
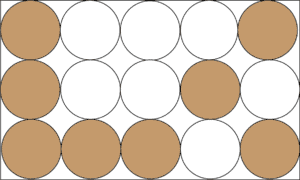
From the model, the brown circles represent sand, while the white circles represent salt. As you can see, the particles of sand and salt are unevenly distributed throughout the mixture.
Can a mixture be separated?
Yes! A mixture can be separated by chemical techniques. Some of these techniques exploit the differences in:
- boiling point (a pure chemical has its own boiling point)
- melting point (a pure chemical has its own melting point)
- freezing point (a pure chemical has its own freezing point) and
- solubility (chemicals can be soluble in some solvents, but insoluble in others)
Some of the common techniques we use in our daily lives to separate mixtures include:
- distillation (takes advantage of differences in boiling points)
- filtration (takes advantage of differences in particle size)
- crystallization (takes advantage of differences in solubility)
- chromatography (takes advantage of differences in solubility)
To separate our sand, salt, and water mixture, we will use gravity filtration to first separate the sand from the salt and water. Next, we will heat the salt solution to evaporate the water from the salt. Now there you have it! You can now boast of having separated a mixture into its pure components.
Check your understanding
- How would you separate a mixture of sand, iron nails, and sea salt?
- In nature most things exist as mixtures. False or True
Click here to grade your answers!