How can we identify matter?
We can identify matter by:
- physical properties
- chemical properties
What’re Physical Properties?
Physical properties are properties we can observe without changing the composition (makeup) of a substance.
What do we mean by without changing its composition?
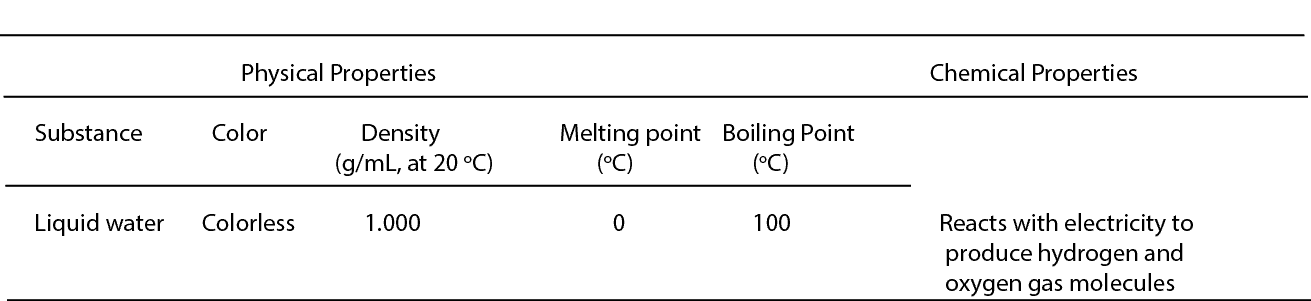
Recall that molecules are made when atoms of the same or different elements react. For example, a molecule of water has the chemical formula H2O, and it is made when molecules of oxygen react with molecules of hydrogen. So the physical properties of water are those properties that can be observed without splitting the water molecule into hydrogen and oxygen atoms.
From this, we can draw an analogy between the physical properties of a pure substance and the physical appearance of a person. For example, to describe a person’s physical appearance, we often look at his or her height, weight, color of hair, skin, and eyes. Similarly, to describe the physical properties of a pure substance, we often start with its macroscopic properties, and these properties include color and texture of the substance.
If two pure chemicals have the same color and texture, how can you identify them?
To identify them, you have to measure some of their other physical properties, and these properties include density, melting and boiling points. Why should you measure density or boiling point to identify a substance? Every pure substance has its own density and boiling point, and so can be used to identify a substance. For instance, many know that a pure substance that freezes at 0 and boils at 100 degrees Celsius is highly likely to be water.
What’re some examples of Physical Properties?
Some examples include:
- color
- texture
- boiling point
- melting point
- freezing point
- density
- taste
- smell
- conductivity
- specific heat
What’re Chemical Properties?
Chemical properties are properties we can observe only when a substance changes its composition. And usually a change in composition occurs when the molecules of the substance reacts with another or energy to make a new substance. Here the makeup of the initial substance changes. As you can see, we can draw an analogy between the chemical properties of a pure substance and the personality of a person. For instance, we often can describe peoples personality by looking at their actions and how they react to situations.
What’re some examples of Chemical Properties?
Some examples include:
- gasoline burns when it reacts with oxygen (flammable), so flammability is a chemical property
- iron reacts with oxygen to form rust, so reactivity is a chemical property
- Some chemicals are corrosive and others not, so corrosiveness is a chemical property
- Some chemicals can be acidic or basic, so acidity and basicity are chemical properties
Here are some physical and chemical properties of water
To learn how to classify matter by state and composition, click here.