What Changes can Matter undergo?
What changes can matter undergo? Matter can undergo two kinds of change:
- Physical change
- Chemical change
What’s a Physical Change?
A physical change is change that can occur without changing the composition of matter. In other words, in a physical change no new substance is formed. Now, we all know water can exists as ice, water, and steam, but in each of these states water is still water, and its chemical composition is still the same, with the chemical formula H2O. To change liquid water to steam, water molecules must only absorb enough energy so that they can break the weak attractive forces between water molecules.
To speed up the process, we often heat our water to boil. In the same way, to convert steam back to liquid water, we must cool our steam to slow down the speed of the water molecules until such a point that they start to attract each other to form liquid water. As you can tell, heating only increase the kinetic energy of water molecules. While cooling only decrease the kinetic energy. So as you change water from liquid to gas, the kinetic energy and the distance between the water molecules increase, while the attractive forces between them decrease. Similarly, as you change gas to liquid, the kinetic energy and the distance between the water molecules decrease, while the attractive forces between them increase.
What’s a Chemical Change?
A chemical change is a change that can only occur with a change in composition of matter. In other words, in a chemical change new chemicals are formed in place of the ones undergoing the change. Chemical changes are all around us. Just look at yourself, you are a complex being in which several chemical reactions or changes are occurring. Thus, the digestion of the food you eat and your aging process are all typical examples of chemical change.
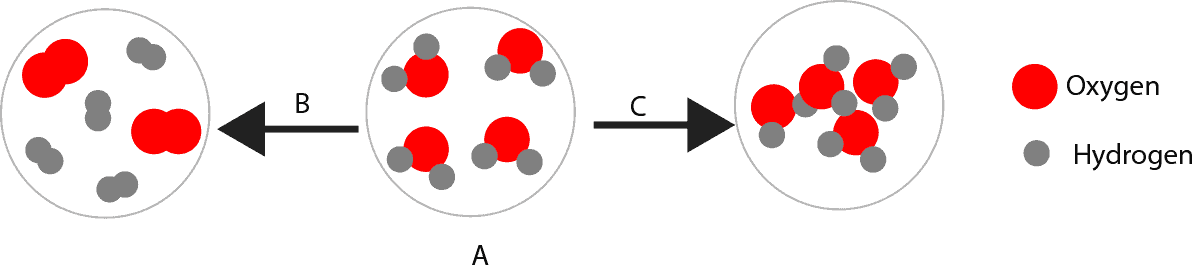
Can you tell whether a Change is Physical or Chemical from a Molecular Picture?
Yes, you can. But you must compare the molecular pictures before and after the change to determine whether the chemical is going through a physical or chemical change.
Check your understanding
- The pictures below represent a molecular view of chemical A going through a physical and a chemical change. Use the diagram below to determine whether path A or C depicts a chemical or a physical change. Explain the reason for your choice.