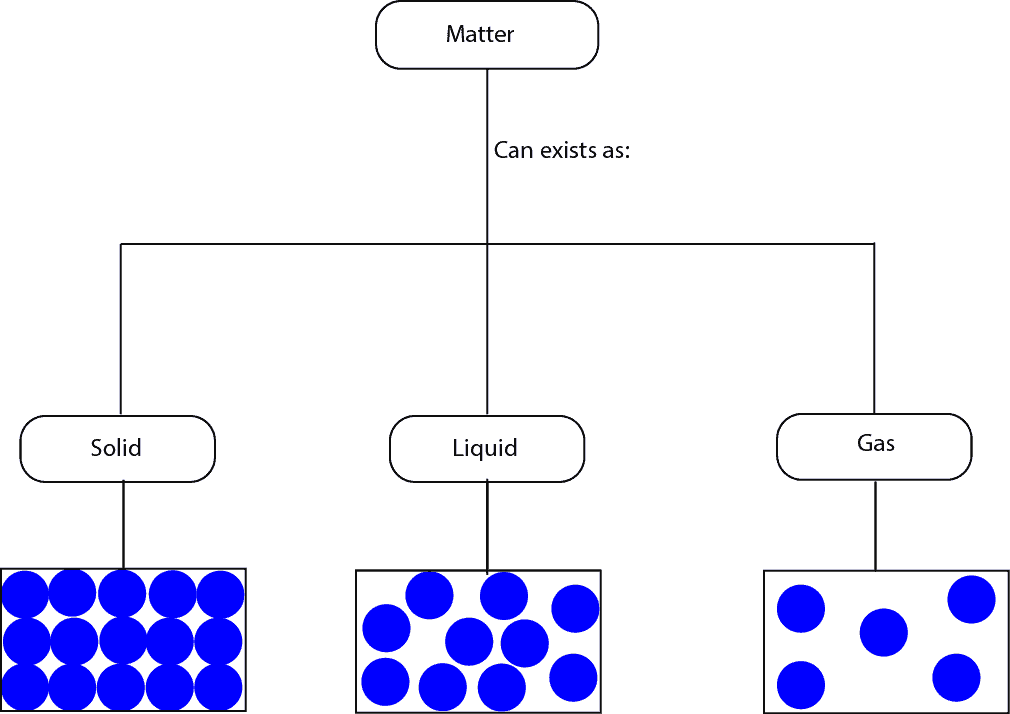
How can Matter be Classified?
Matter can be classified in two ways:
- State
- Composition
How to classify matter by state
Matter can exists as solid, liquid or gas. Here is a model showing the three states
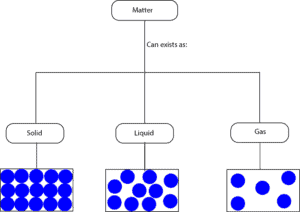
From the particle models, you can see that for:
- solids the molecules are closely packed, as a result, they can only vibrate from their fixed positions
- liquids the molecules are not closely packed as in solids, as a result, they can move around much more than the molecules in solids
- gases the molecules are widely spaced, as a result, they can move about much more random
Why’s it that in solids the molecules are tightly packed?
Because the attractive forces between the molecules are much stronger than their kinetic energy.
Why’s it that in liquids the molecules are loosely packed?
Because the kinetic energy of molecules is slightly stronger than the attractive forces between them.
Why’s it that in gases the molecules are widely spaced?
Because the kinetic energy of the molecules is much stronger than the attractive forces between.
How to classify matter by composition
Matter can be classified as mixture or pure substance. When matter is a mixture, it can either be homogeneous or heterogeneous mixture. When matter is a pure substance, it can either be a compound or an element. Here is a model showing how matter can be classified by composition.
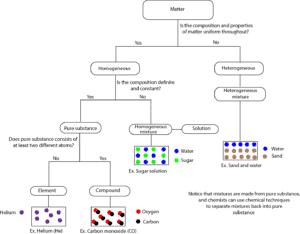
From the flow diagram, a piece of matter is examined to determine whether it is homogeneous or heterogenous. If it is heterogeneous, then right away it is a heterogeneous mixture. However, if it is homogeneous, then it can either be a pure substance or a homogenous mixture. If you determine that its composition is fixed, then it is a pure substance. And this pure substance can either be an element or a compound. Notice from the model that a mixture like sugar solution can be homogeneous, but not a pure substance.
Here is the above diagram drawn slightly different.
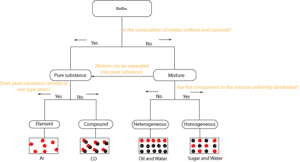
As you can see, you can ask right away whether matter’s composition is uniform and constant throughout. If it does, then it is a pure substance. If not, it is a mixture.
To learn more about pure substance, click here and about mixture, click here.