What’s a Pure substance?
A pure substance is a chemical that has definite and constant composition. Definite composition means that the makeup of the chemical is fixed and constant throughout the pure substance. Another way of saying it is that a pure substance has the same type of chemical and this chemical has the same composition throughout the substance.
Let’s use the model below to illustrate the concept.
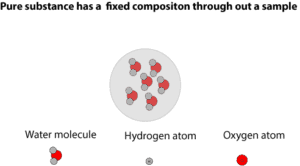
From the model, you can see that it consists of only water molecules. Each water molecule consists of two hydrogen atoms bonded to one oxygen atom in a ratio of 2 hydrogen atoms to 1 oxygen atom. So, the amount of hydrogen or oxygen in pure water will always be the same no matter its source.
That’s the beauty of the law of constant composition—- atoms combine in fixed mass ratio.
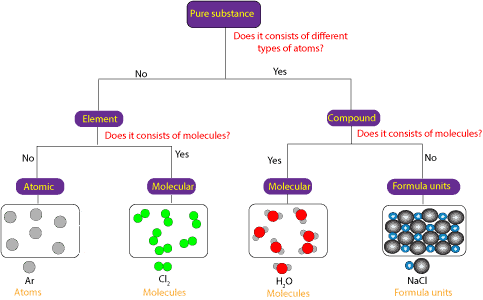
A pure substance can consists of only:
- atoms
- molecules or
- formula units
Here is a model showing the difference between pure substances that are atomic, pure substances that are molecular, and pure substances that consist of formula units;
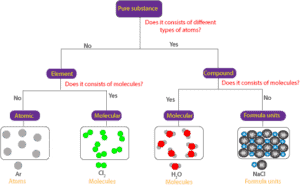
From the concept map, you can see that a pure substance can consists of different or the same type of atoms. If the pure substance consists of the same type of atoms, then it is an element. If the pure substance consists of different types of atoms, then it is a compound. If the element consists of individual atoms, then it is atomic, and if it consists of molecules, then it is molecular.
In the same way, if the pure substance is a compound, and the compound consists of molecules, then we can say that the compound is molecular. And if the compound consists of ions held together in a repeating pattern, then we can say that the compound consists of formula units.
Formula units are common in ionic compounds, while molecules are common in covalent compounds.