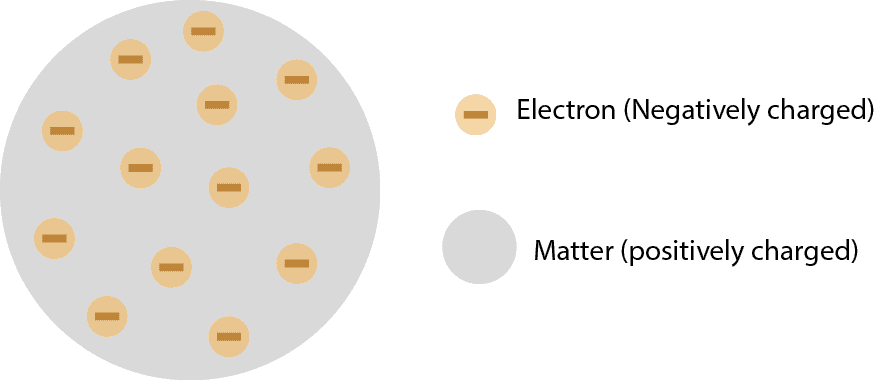
What’s the atomic theory?
The atomic theory is based on the finding that all matter consists of invisible particles called atoms.
What laws help develop the Atomic theory?
These laws are:
Law of conservation of mass
This law means the mass of reactants before chemical change (chemical reaction) is always equal to the mass of products after chemical change. In otherwords, if hydrogen reacts with oxygen in an air tight vessel to make water, the mass of reactants (hydrogen and oxygen) is always equal to the mass of product (water).
How did chemists determine the mass of the reactants and products?
They simply weighed the mass of the reactants before the reaction and the mass of the products after the reaction.
Law of constant composition
This law states that the composition of a pure compound will always be the same regardless of its source. How can that happen? It happens because a fixed mass of one atom always combine with a fixed mass of another. As a result, regardless of its source, a pure compound will always have the same composition, thus, the mass of the elements in it will aways remain fixed. For example, water obtained from melting ice, condensing steam, and reacting hydrogen with oxygen will always have the same mass of hydrogen (11.2%) and oxygen (88.8%) in it.
How did chemists calculate % composition of hydrogen and oxygen?
They first passed electricity through water, thus, splitting water molecules into hydrogen and oxygen gas. These gases where then collected and measured and their percentage composition calculated.
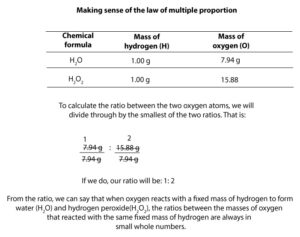
Law of multiple proportions
This law states that when an element, say B reacts with another element, say A to form more than one type of compound, say A2B and A2B2, then, the masses of B that reacted with a fixed mass of A will always form a ratio that can be expressed in small whole numbers to each other. Let’s clarify this statement.
Imagine that you are running two reactions in two different containers. Let’s say in container one, 1.0 g of hydrogen reacts with 7.94 g of oxygen to form water (H2O). In container two, another 1.0 g of hydrogen reacts with 15.88 g of oxygen to form hydrogen peroxide(H2O2). Since the mass of hydrogen is fixed, we can write a ratio between the mass of oxygen in container one to the mass of oxygen in container two. If we do, we will get: 7.94 : 15.88. Now, if we divide through by the smallest ratio 7.94, we will get: 7.94/7.94 : 15.88/7.94 = 1:2. So, the (1:2) is the ratio between the masses of oxygen that will react with a fixed mass of hydrogen to form water and hydrogen peroxide. What this law is saying is that atoms always react whole, and they are always in a ratio of small whole numbers to each other. These ratio of small whole numbers include: 1:1, 1:2, 2:1. Here is a table showing the above calculation:
To explain the reasons behind the three fundamental laws, Dalton proposed his atomic hypothesis which later became the first atomic theory.
Check your understanding
- What’s the difference between Rutherford and Thomson’s atomic model?
Click here to grade your answer.