What major factor can affect the changes matter undergo?
The major factor that can affect changes matter undergo is Energy.
How does Energy interact with matter?
No-one knows exactly what energy is, but it is surely the non physcial component of the universe that make things go. Scientists can track the flow of energy as it travels through matter. For example, as we burn gasoline in our cars it causes the car’s engine to move and this movement causes the car’s wheels to turn and transport us from place to place. When such chemicals have the power to do work, we usually say they possess energy. Since the energy is only available when gasoline undergoes a chemical change, we general say that gasoline generates chemical energy.
What then is chemical potential energy?
Chemists often say that chemical potential energy is energy stored in the bonds of molecules.
How is this energy stored in the bonds of molecules?
Recall that a molecule is formed when two or more atoms make a bond. That is these atoms are held together in a mechanical arrangement by an invisible force. This invisible force holding these atoms together is what we call chemical potential energy.
How does this chemical energy get release?
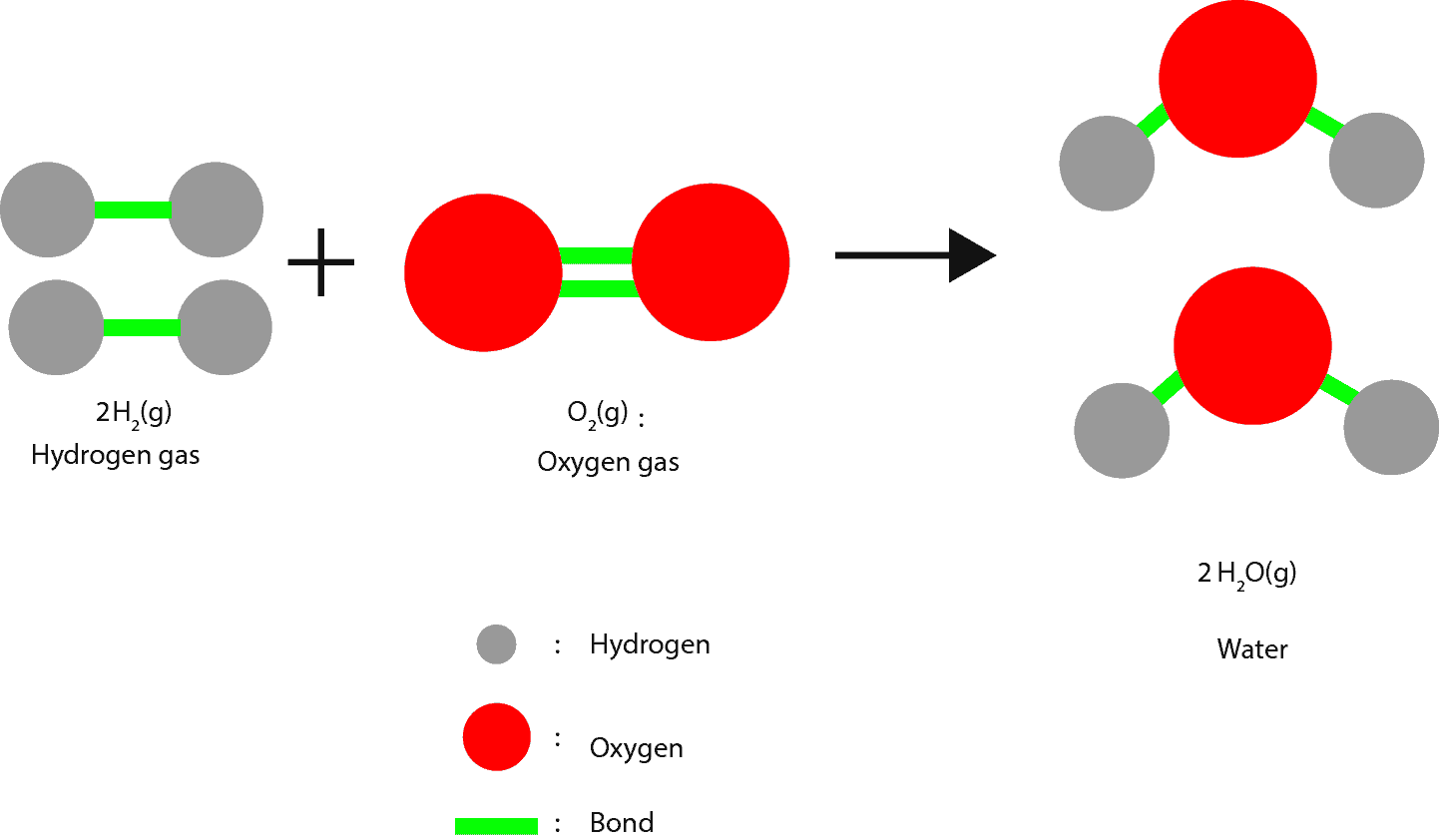
This energy is usually released as heat when these atoms or molecules separate from their initial arrangement and regroup in other ways. When the regrouping results in a change in composition, chemists usually call the process a chemical change or chemical reaction. When the regrouping results in no change in composition, chemists usually call the process a physical change. Let’s use this molecular model to illustrate regrouping in a chemical change.
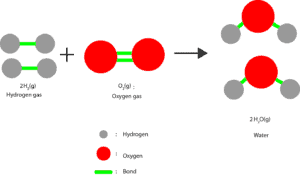
As we can see, the green lines represent the bonds. To form water, the bonds in the oxygen and hydrogen molecules must be broken so that the hydrogen and oxygen atoms can unite (regroup).
How do bonds break?
At the molecular level, these gas molecules are constantly moving (kinetic energy) and colliding with one another. Some of these collisions can be strong enough to break the bonds within oxygen and hydrogen molecules. As a result, hydrogen and oxygen atoms can unite to make water.
From these collisions, we can tell that energy is always put into atoms or molecules to break bonds. On the other hand, when bonds form some of this energy must be released from these atoms or molecules to the surroundings.
If more energy is released than is required, the excess energy is transferred to the surroundings as heat. So from the above reaction when hydrogen gas reacts with oxygen gas lots of energy is released to the surroundings. Because the bond made between hydrogen and oxygen atoms (red and grey balls) are much stronger than those in hydrogen molecules and oxygen molecules.
Now let’s use the molecular model below to illustrate regrouping in a physical change
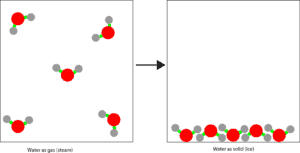
The water molecules in the left box represent water molecules in steam (gaseous state). While the ones in the right box represent water molecules in ice (solid state). The water molecules in steam are widely spaced and move at random. While the water molecules in ice are closely packed and vibrate in their fixed positions.
How do we go from steam to ice?
We go from steam to ice by cooling our steam until it turns to ice.
At the molecular level, what is happening to the water molecules as we cool steam?
We are slowing the speed of the water molecules by lowering the temperature until such a point that they start to attract each other and regroup.
So where did all that energy in steam go?
It was transferred from the steam to the surroundings as heat. If we want to get back our steam, we have to put in that same amount of energy to heat water to steam.
And where do we get this energy to heat our water?
We usually get this energy in our homes by burning natural gas or from electricity generated by hydroelectric or coal power plants.
From our discussion, we can tell that when one substance loses certain amount of energy, another substance gains an equivalent amount. So we can confidently say that we can’t create or destroy energy, but we can only transform it from one form to another. This transformation does not mean that the energy in itself changes, it just means that the energy appears in a different vehicle. From these last two sentences, we can state an important law of nature called the Law of Conservation of Energy. This law state that while we can transform energy from one form to another, we cannot create or destroy it.
Notice! The law of conservation of energy is not the same thing as energy conservation. Energy conservation basically means that we should use our energy resources wisely so that these resources will be there for future generations.