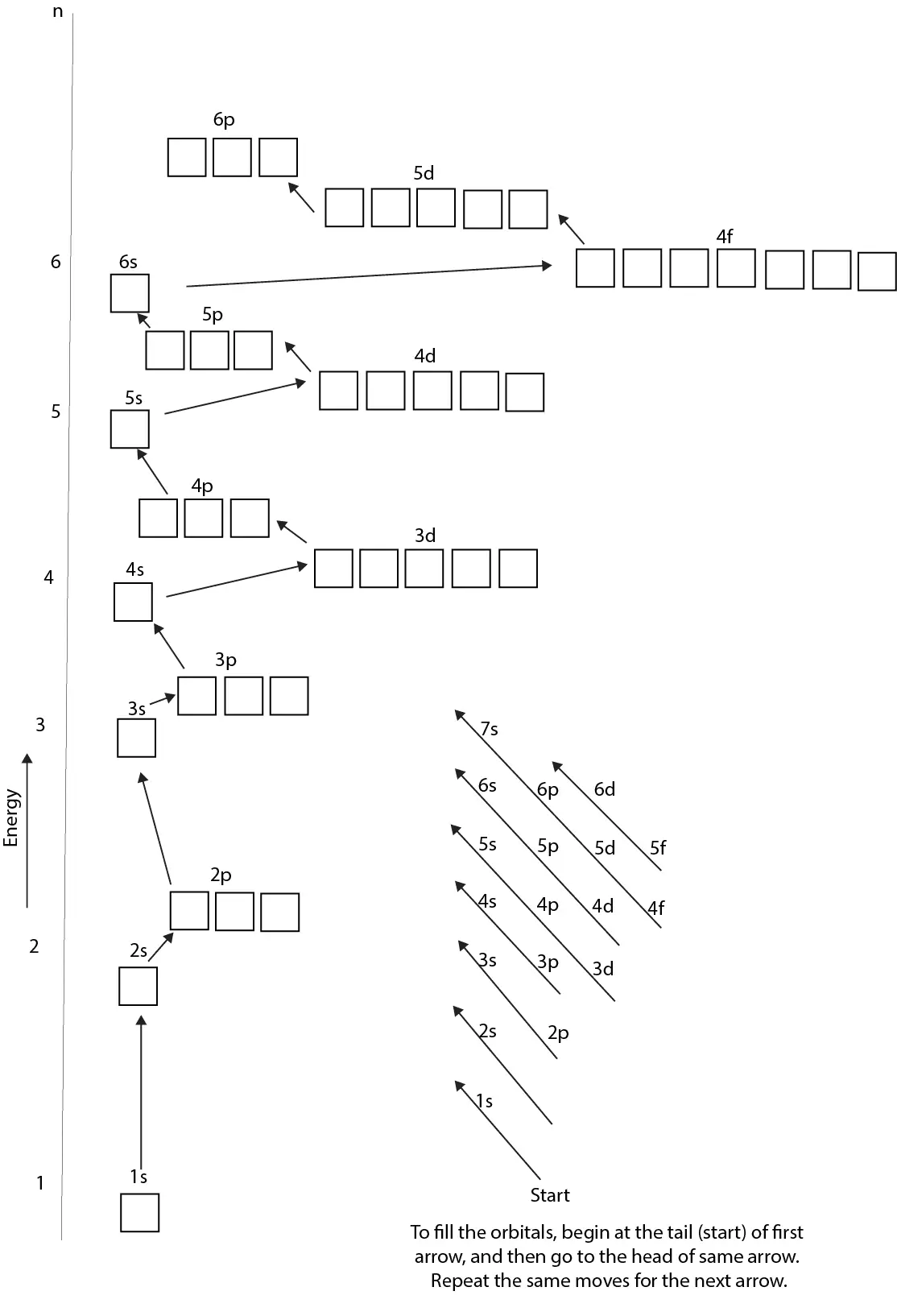
How’re the orbitals in an atom arranged?
The orbitals in an atom are arranged according to energy levels, and in these energy levels the orbitals are ordered s, p, d, and f. And the higher the energy level, the more orbitals it has. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. As the energy level increase in distance from the nucleus, so does its level of energy, with the energy levels at the top having the highest energy.
Meaning an electron in an n = 1 energy level has less energy than an electron in an n = 5 energy level. You will also realize that as you move up from the nucleus of an atom, the orbitals are clustered together into groups with some of the orbitals crossing each other (example the 4s and 3d). Generally, orbitals of a particular group have about the same average distance from the nucleus. And since they have about the same average distance, they should have about the same energy. For these reasons, orbitals that have the same energy are usually referred as being in the same Energy level.
To determine how many orbitals are in an energy level, you simply multiply the number of the energy level by itself. For example, to determine the number of orbitals in n = 3 energy level, you simply multiply 3 by itself. Thus, 3*3 = 9 orbitals. We can represent this calculation in a simple mathematical formula as: number of orbitals = n2, where n is the number of the energy level, and n can take values only from 1 through 7.
What is the maximum number of electrons that can go in each energy level?
To determine the maximum number of electrons that can go in each energy level, you simply multiply n2 by 2. Thus,
maximum number of electrons = 2n2
where the 2 in front of the n2 represents the maximum number of electrons that can go in a particular type of orbital, and n the number of the energy level. Thus, the maximum number of electrons that can go in the n = 3 energy level is:
2 (3)2 = 18 electrons
As you can see, the same type of calculation can be done for the rest of the energy levels. The only number that will change in the calculation is the number of the energy level. To read how chemists fill atomic orbitals with electrons, click here.