What’s the valence of an atom?
Valence determines how many atoms of an element a particular atom can combine with. Now let’s explain the previous statement with examples of compounds we all know:
HCl: hydrochloric acid
H2O: water
H3N: ammonia and
H4C: methane
In HCl, the hydrogen and chlorine atoms combine in a ratio 1 to 1. How do you know this from the chemical formula? You know this because when atoms combine atom for atom the subscripts of the atoms in the chemical formula also exist in a ratio of 1 to 1. As a result, if no subscripts are shown in the chemical formula, it usually means that the atoms combine atom for atom.
In H2O, you can see that hydrogen and oxygen atoms combine in a ratio of 2 hydrogen atoms to 1 oxygen atom.
In H3N, you can see that the hydrogen and nitrogen atoms combine in a ratio of 3 hydrogen atom to 1 nitrogen atom.
To determine the combining power (valence) of an element, we have to choose one element as the standard to which we can compare other elements to it. Fortunately, hydrogen atom cannot combine with more than one atom of any other element. For this reason, chemists chose it as the standard and assigned it a valence of 1. So what’s valency? Valency is the combining power of an element, and it determines how many atoms of an element a particular atom can combine with. As a result, in H2O, oxygen (O) has a valence of 2 ( recall that hydrogen is assigned a valence of 1).
In H3N, the valence of nitrogen (N) is 3, and in H4C the valence of carbon is 4.
Can you tell the valence of an element from its electronic configuration?
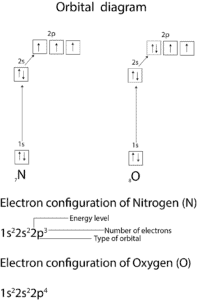
Not always! To determine the valence of nitrogen from its electron configuration, you have to count the number of unpaired electrons occupying the highest energy levels. Let’s use the model below to determine the valence of nitrogen (N) and oxygen(O).
From the orbital diagram of N, the 2p orbital has three unpaired electrons (valence of 3) which it can use to combine with hydrogen to make ammonia (H3N).
Similarly, oxygen has two unpaired electrons (valence of 2) in the 2p orbital which it can use to combine with hydrogen to make water (H2O).
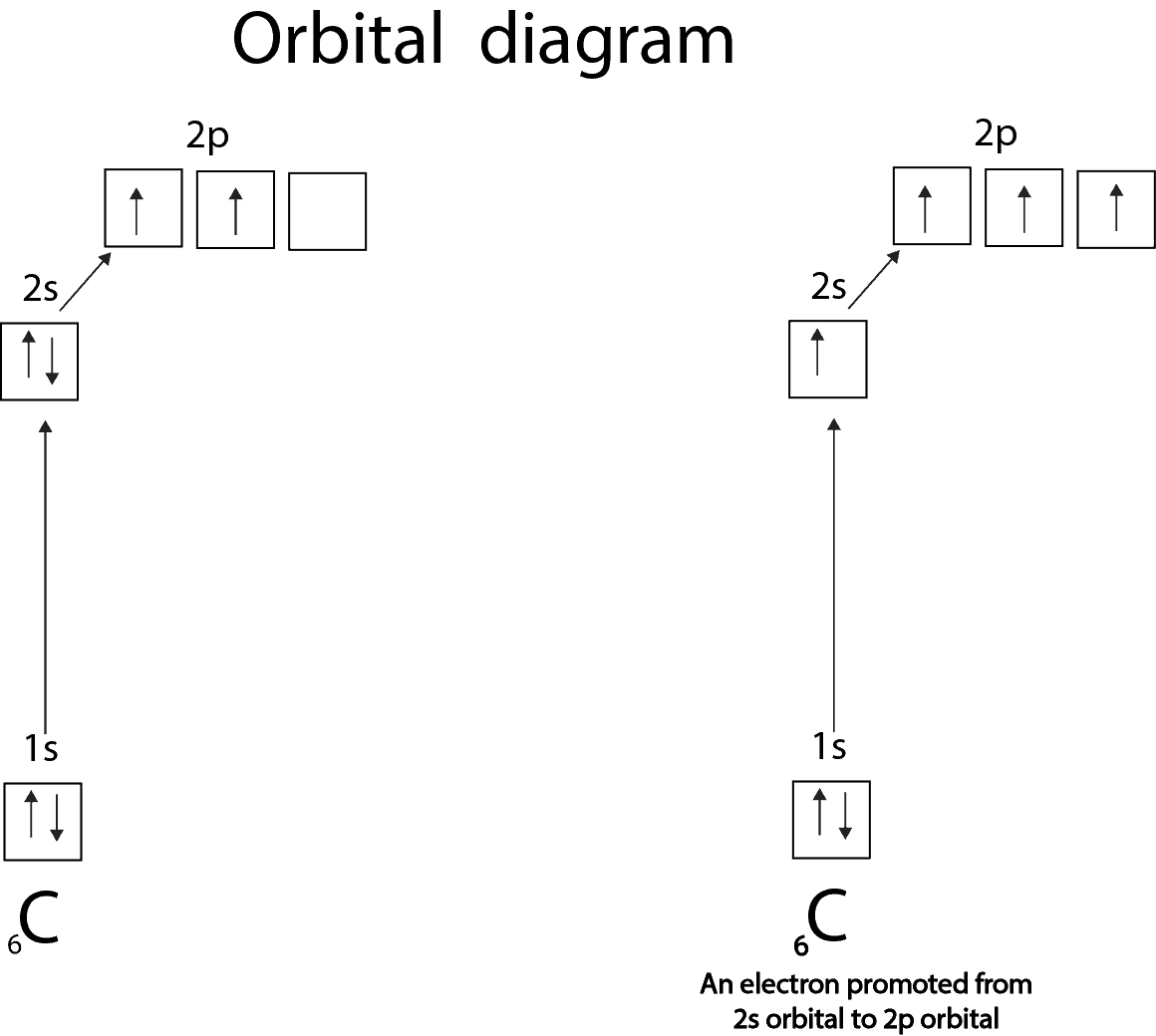
Sometimes the valence is not obvious from the electron configuration. Let’s use carbon to illustrate this.
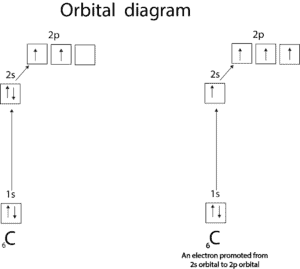
From the orbital diagram on the left, you can tell that carbon has only two unpaired electrons, therefore has a valence of 2. However, in the compound CH4, one atom of carbon combines with 4 atoms of hydrogen. So how does carbon do that? Carbon is able to make CH4 because one of the 2s electrons is promoted from the 2s to the 2p orbital. This promotion results in four unpaired electrons which then can combine with 4 atoms of hydrogen to make CH4.
Finally, an element that has a valence of 1 is called univalent, 2, divalent, 3, trivalent, and 4, tetravalent. Usually, chemists can find the valence of any element by relating it to how many atoms of hydrogen it can combine with or to an element that reacts with it and has its valency already determined.
What’re valence electrons?
Valence electrons are electrons that occupy the highest energy level. These electrons are usually available to combine (bond) with other atoms. To determine the valence electrons in nitrogen (N), you must count the electrons in the highest energy level (n = 2, thus, count the electrons in the 2s and 2p orbitals). If you do, the valence electrons of N is 5. For oxygen (O), is 6, and for carbon (C) is 4.
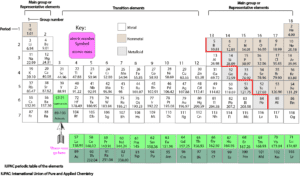
Can the periodic table tell how many valence electrons an element has?
Yes! From the IUPAC periodic table numbering system, the valence electrons of an element can be determined by reading the last digit of the group number at the top of the column (keep in mind that since group one and two have single digits, these digits will represent the last digit of the column). However, there is an exception. Only elements in group 10 can have ten valence electrons. Here is the periodic table showing the details.
.