Why do atoms bond with one another?
Atoms bond with one another so that they can lower their energy and become stable. And as atoms bond with other atoms, they often make molecules with unique chemical and physical properties. Let’s use the model below to explain how atoms bond to become stable.
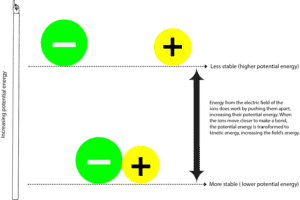
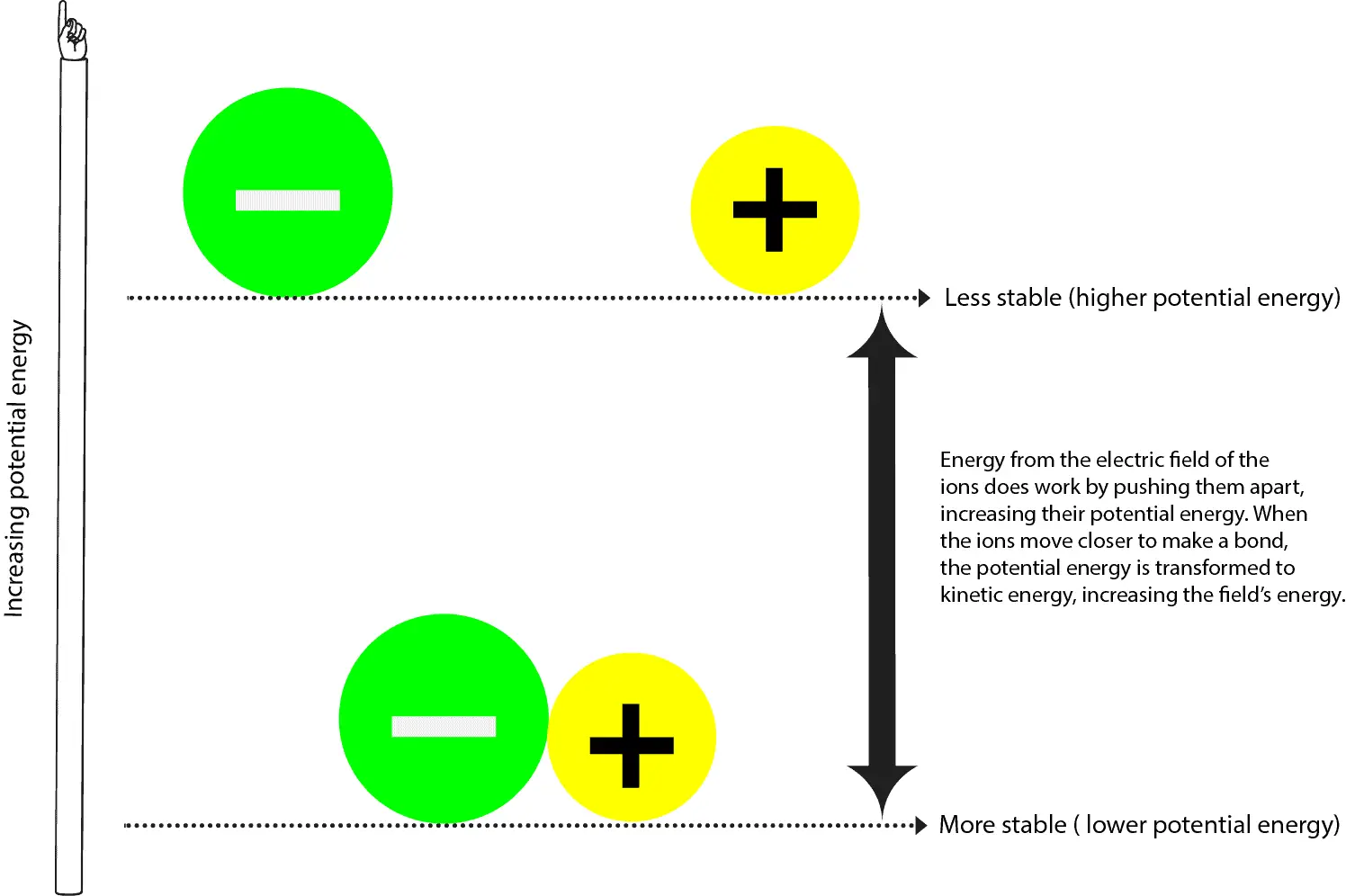
When the negatively charged atom (anion) and the positively charged atom (cation) form a bond, they are usually more stable with lower potential energy. Potential energy is the energy due to the relative positions of these ions in the electric field surrounding them. Electric field is an area in which a force exerts itself. In some ways, an electric field is similar to a magnetic field. And you can see these lines of force surrounding a magnetic when you spread iron filings around it.
To separate the negative ion from the positive ion, the electric field surrounding the ions must do work by pulling these ions apart. As the electric field do this, it also transfers energy to these particles, increasing their potential energy. You can relate this process to you pulling two magnets that are attracted to each other. In this case, you are the force field suppling the energy to pull them apart, and as you pull them apart, the magnets increase their potential energy. And because they have to do more work to stay away from each, they become unstable. As soon as you let go, the two magnets are quickly attracted to each other, lowering their potential energy. Now,
where did the rest of the potential energy go?
That energy was transferred back to you and the surroundings in the form of kinetic energy. Kinetic energy is the energy an object has when it moves, and we often feel this energy as heat. So anytime you feel heat, it means that energy is moving from a hotter place to a colder place. Or put in another way, from a more concentrated to a less concentrated source. Keep in mind that energy can never be transferred the other way around— from a colder to a hotter place.
So as energy is transferred from and back to the field or from and back to the universe, we usually say that energy is conserved. Meaning we can’t create or destroy energy, we can only convert it from one form to another. That’s from potential to kinetic energy and from kinetic to potential energy.
What do atoms use to bond with other atoms?
Atoms use their valence electrons to bond with other atoms. And for atoms to bond, they must do at least one of the following;
- gain electrons from other atoms
- lose electrons to other atoms
- share electrons with other atoms
And recall that valence electrons occupy the highest energy orbital in an atom. And you can visualize these electrons when you write the electron configuration for an atom. If you do with the main group elements from the first and second period, you will notice that hydrogen has one valence electron, nitrogen has 5, oxygen has 6 and neon has 8.
If we draw these valence electrons by putting dot(s) on the top, right, bottom and left side of their chemical symbols, we will get these simple diagrams called Lewis dot diagrams, named in honor of Lewis, who was first to use them to explain the idea of bonding. Now, let’s further discuss bonding with the following Lewis structures:
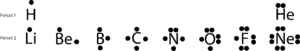
If you look at the elements on the second period, you will notice that only Neon (Ne) has two electrons on each of its four sides. If you sum all the electrons on all the four sides, you will discover that there are eight electrons surrounding it.
The number eight is tied to the last digit of the group number Ne belongs, group 18. This special group of elements, called the noble gases, tend to be stable and un-reactive under normal conditions. With the exception of helium, they all have eight valence electrons in the group.
Because of their chemical behavior, Lewis was able to deduce that in order for the other elements to achieve a stable configuration like the noble gases, they must react with other atoms to achieve it. From this conclusion, Lewis proposed the octet rule. The rule of eight.
But there are exceptions, as you can tell, hydrogen only needs two electrons to achieve a stable configuration similar to He. Despite this and other shortcomings, Lewis dot structures can explain how atoms bond in a simple way without us drawing complex atomic orbitals.
What causes atoms to bond with other atoms?
Energy! Energy is usually the driving force behind bonding. For atoms to bond, atoms must strike a delicate balance between the attractive and repulsive forces of their nuclei and electrons. To learn how two hydrogen atoms share electrons to create the hydrogen molecule, click here.