Can a cation (positively charged ion) be larger than its neutral atom?
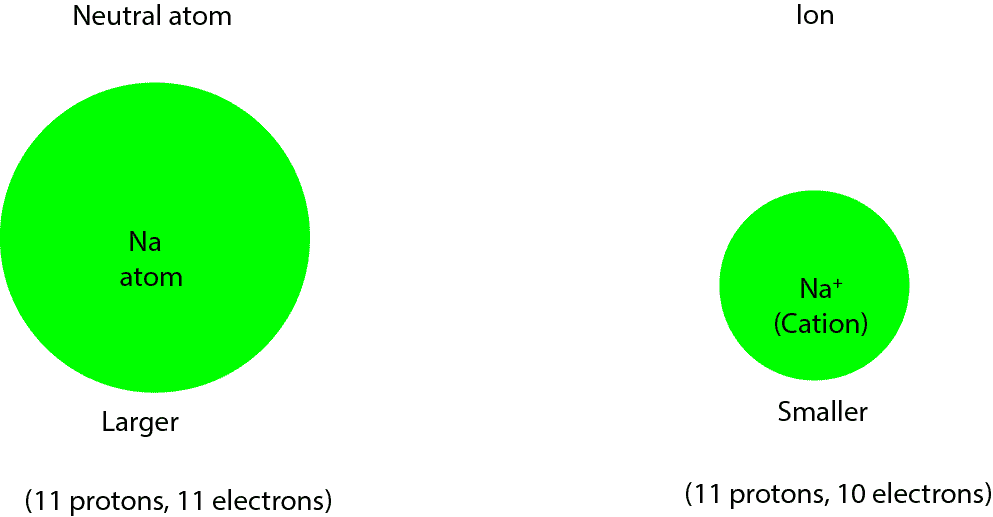
No! A cation is usually smaller than its neutral atom. Let’s explain this further with an example. Given neutral sodium atom (Na) and sodium plus one ion (Na+), which is larger? First, let’s figure out the number of protons and electrons in neutral Na and its cation, Na+.
Na: number of protons: 11; number of electrons: 11
Na+: number of protons: 11; number of electrons: (11 electrons – 1 electron) = 10 electrons. Recall that the +1-positive charge means that an electron has being removed from neutral sodium to make Na+ ion, and when the size of charge is 1, we usually omit 1 when we write the chemical symbol for the ion: Na+.
Now let’s determine the proton-electron ratio for Na and Na+
Na: 11 protons and 11 electrons. Here the number of protons are as many as the number of electrons.
Na+: 11 protons and 10 electrons. Here the number of electrons are fewer than the number of protons. Since protons carry a positive charge, and they are more than the number of electrons in the Na+ ion, they will pull in the electrons and their orbitals much closer to the nucleus, shrinking the size of the Na+. Thus, the Na+ ion will be smaller than its neutral form, Na. Here is a model showing the differences in size.
Can an anion (negatively charged ion) be larger than its neutral atom?
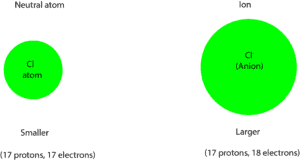
Yes! Let’s explain this with an example. Given neutral chlorine atom(Cl) and chloride ion (Cl–), which is larger? In the same way, let’s figure out the number of protons and electrons in each:
Cl: number of protons: 17, number of electrons: 17.
Cl–: number of protons: 17, number of electrons: 17 electrons plus 1 electron = 18 electrons. Recall that (-1) means that one electron has been added to the chlorine atom.
Now let’s determine the proton-electron ratio for Cl and Cl–
For neutral Cl, the number of protons are equal to the number of electrons: 17 protons; 17 electrons.
For Cl–, the number of protons are fewer than the number of electrons: 17 protons; 18 electrons. Since the protons are fewer, they can’t strongly pull in the electrons and their orbitals closer to the nucleus, as a result, the size of the chloride ion (Cl– ) will be larger than the size of its neutral atom (Cl). Here is a model showing the differences in size.