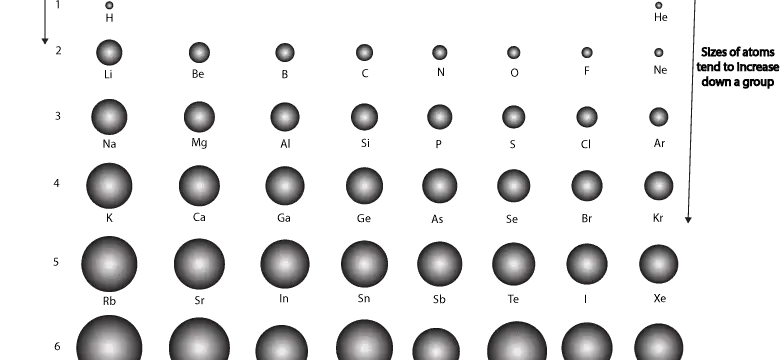
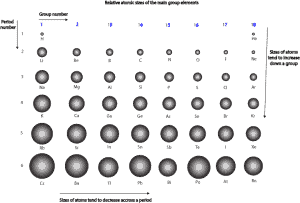
How does atomic size vary across and down the periodic table for main group or representative elements?
For main group elements, atomic size gets larger as you go down a group (column) and atomic size gets smaller as you go across a period (row). Let’s use the following condensed periodic table to show the relative sizes of the main group elements.
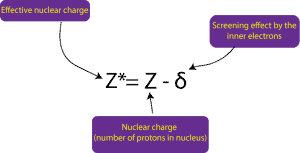
Why do atoms get larger as you go down a group?
As you go down a group one atom at a time each step puts valence electron(s) in the highest and largest shell called energy level. In addition to that the electrons buried deep inside the atom (inner electrons) usually repel the outer electrons. This repulsion, called screening by the inner electrons usually cause the nucleus to attract the outer electrons less strongly, resulting in an increase in the size of the atom. Another way of saying the same thing is that as you move down a group, the size of the atoms increase. The size increase because the effective nuclear charge (positive charge of nucleus) experienced by the outer electrons decreases down a group. Effective nuclear charge decreases because the inner electrons repel the outer electrons, weakening the nucleus pull for the outer electrons. We can write a relationship that describes the effective nuclear charge as:
Where Z* is the effective nuclear charge, Z, the nuclear charge, and δ, the screening effect by the inner electrons.
For instance, if you look for lithium on your periodic table, you will notice that it has an atomic number of 3. And since Li is neutral, it immediately follows that, it has 3 protons and 3 electrons. And we can write its electron configuration as :1s22s1
From the electron configuration notice that Li has only one electron in the 2s orbital and 2 inner electrons in the 1s orbital. The two inner electrons in the 1s weakens the nucleus (3 protons) attraction (Z) for the one electron in the 2s orbital. Because of this, we can predict the effective nuclear charge (Z*) experienced by the electron in the 2s orbital by subtracting the two inner electrons from the number of protons. That is: 3 – 2= +1, where 3 is the number of protons (nuclear charge) and 2, the inner electrons. As you can see, the +1 effective nuclear charge experienced by the 1s electron is far less than the nuclear charge.
Why do atoms get smaller as you go across the period?
As you go across a period from left to right and atom to atom, each step adds another electron to the same energy level (shell) and a proton to the nucleus. The added electron is slightly repelled by the other electrons in the shell, while the added proton increases the positive charge of the nucleus. This increase in nuclear charge cause the nucleus to attract the electrons and their orbitals more strongly, shrinking the size of the atom.
How can atomic size be measured?
If we consider an atom to be a sphere, we can describe its size by its atomic radius. And atomic radius can be measured by x-ray diffraction. A technique in which atoms inside a crystal scatter x-rays. For a particular substance, the pattern of scattering is usually unique, and it can be used to deduce the size of an atom.
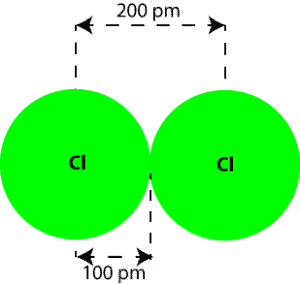
For instance, in a molecule such as Cl2, in which both atoms are identical, we can define atomic radius (covalent radius) as one-half of the internuclear distance. This is the distance from the nucleus of each atom to the dotted line drawn between the two circles. Here is an illustration of atomic radius and internuclear distance:
From the illustration, the internuclear distance is 200 pm, while the atomic radius (covalent radius) is 100 pm, which is calculated by dividing the internuclear distance by 2. That is 200 pm/2 = 100 pm