What’s an ionic bond?
An ionic bond is a force of attraction between a positive and a negative charged ion. These oppositely charged ions are usually produced when a metal transfers its electron to a nonmetal. For example, when an electron is removed from a neutral sodium atom, the sodium atom becomes a positively charged sodium ion (Na+), and when this electron is transferred to a neutral chlorine atom, the chlorine atom becomes a negatively charged chloride ion(Cl–). Since opposite charges attract, the sodium and chloride ions will be held together by a strong attractive force called an electrostatic force. Chemists, however, call this electrostatic force an ionic bond.
Generally, ionic bonds form between metals and nonmetals. The metals usually have low ionization energy, while the nonmetals have high electron affinity.
Why does it form between metals and nonmetals?
It takes much less energy for metals to give away electrons. For instance, it takes much less energy for a sodium atom to give away its valence electron to achieve an octet than it is to share or receive electrons from another atom to achieve it.
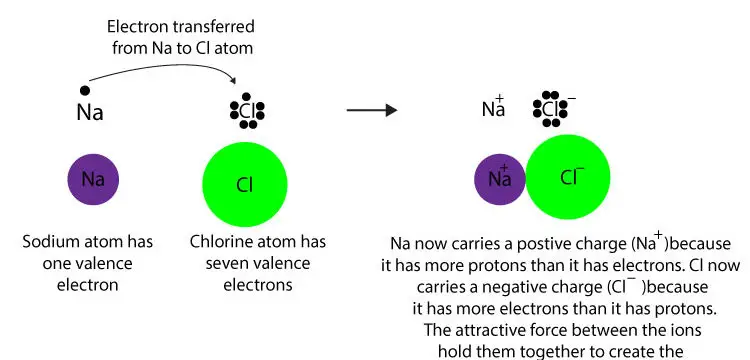
Let’s use the models below to explain how sodium chloride is made.
After Na loses an electron, its electron configuration becomes: 1s22s22p6. As you can tell, Na+ now has 8 valence electrons in its highest occupied energy level (2s22p6). Similarly, after Cl receives an electron, its electron configuration becomes: 1s22s22p63s23p6). As you can see, Cl– now has 8 valence electrons in its highest occupied energy level (3s23p6).
Although some atoms share electrons to form covalent bonds and others transfer electrons to form ionic bonds, these bonds are still similar in strength because they both involve a force of attraction between unlike charges.
Compounds that are formed as a result of ionic bonding are usually called ionic compounds. These compounds do not exist as molecules. They exist as formula units, where the ions are arranged such that the positive ions are adjacent to the negative ions in a repeating three-dimensional pattern.