How do atoms form covalent bond?
Atoms form covalent bonds when two or more nonmetals share valence electrons.
What’s a covalent bond?
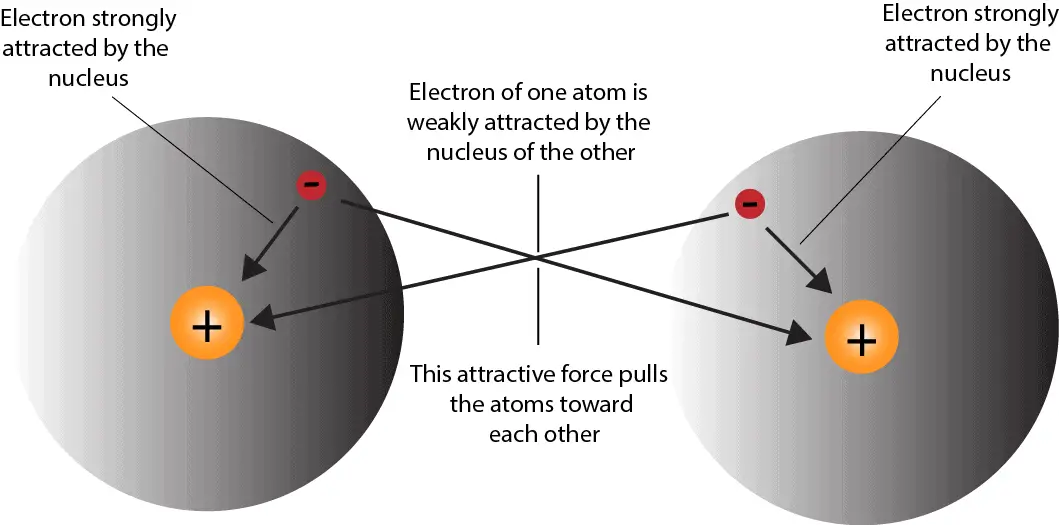
A covalent bond is a force of attraction between two or more atoms.
How do atoms share valence electrons?
They share electrons by pooling their valence electrons between the nuclei of the atoms involved. Generally, covalent bonds form between nonmetals.
Why do covalent bonds form between nonmetals?
Non metals form covalent bonds in order to achieve a stable electron configuration similar to that of the noble gases.
Is energy always released when covalent bonds form?
Yes! Energy is always released when bonds are made. As the atoms collide with each other, some of the energy involved in the collision is usually released to the surroundings as heat. Conversely, that same amount of energy must be supplied to break the bond. As an analogy, a bond is a force similar to the force of attraction between two magnets. Just as energy is always required to pull two magnets apart, energy is also required to pull bonded atoms apart (break a bond).
To learn how
- hydrogen atoms form covalent bond, click here
- chemists apply Lewis dot diagrams to explain bonding in simple molecules, click here
- atoms share electrons unequally to form polar covalent, click here
.