What’s a chemical bond?
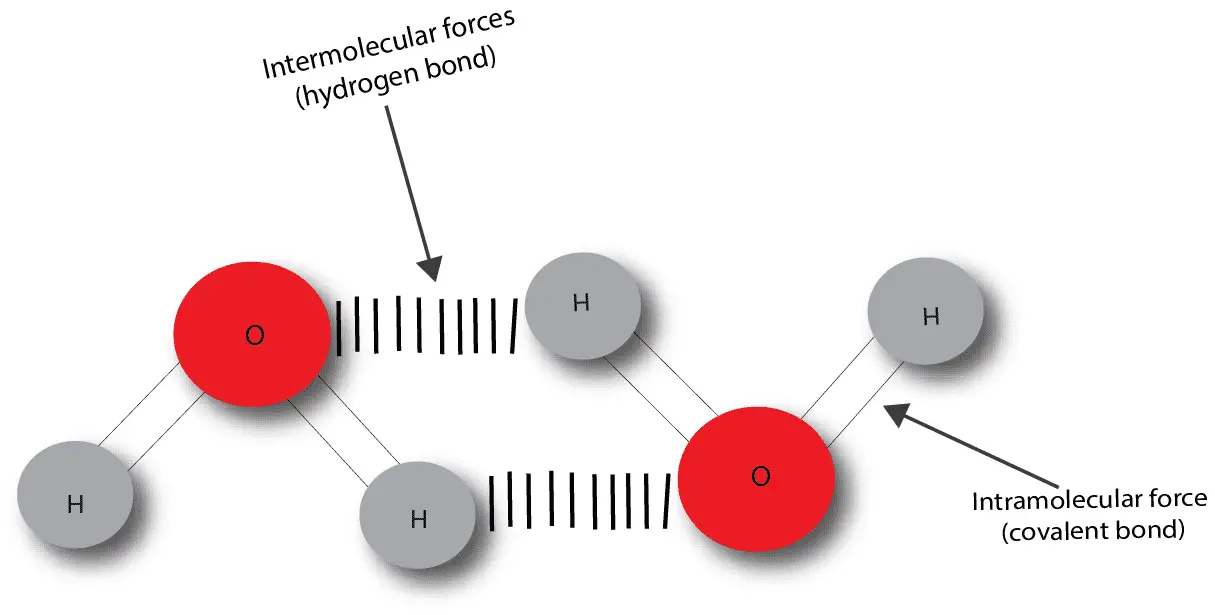
A chemical bond is an attractive force between two or more atoms. This attractive force can be intermolecular or intramolecular. The intermolecular forces are weaker and exists between molecules to hold the molecules together, while the intramolecular forces are stronger and exist within molecules to hold the atoms together. Let’s use a model consisting of two water molecules to point out the difference between inter- and intra-molecular forces.
As you can see, intramolecular forces are the forces that hold atoms together to make a molecule. During a chemical change (chemical reaction) these forces are the ones that usually gets broken to allow the atoms to regroup to form a new substance. We know of two ways in which intramolecular forces can exist. One is called the covalent bond and the other is called the ionic bond. If you want to learn more about covalent bond, click here. And if you want to learn more about ionic bond, click here.
What then are intermolecular forces?
Intermolecular forces are the forces that hold two or more molecules together. During a physical change these are the forces that usually gets weakened or strengthened to allow a substance change from one state to another. For example, when ice is heated, it can change from solid to liquid and to steam. In the same way, if the steam is contained and cooled, it can change back to liquid and solid.
We know of two ways in which intermolecular forces can exist. One is called the Vander Waals forces and the other dipole-dipole forces. Dipole-dipole forces are stronger and are the attractive force between polar molecules, while Vander Waals forces are weaker and are the attractive force between nonpolar molecules. If you want to read more about Vander Waals forces, click here, and about dipole-dipole forces, click here.