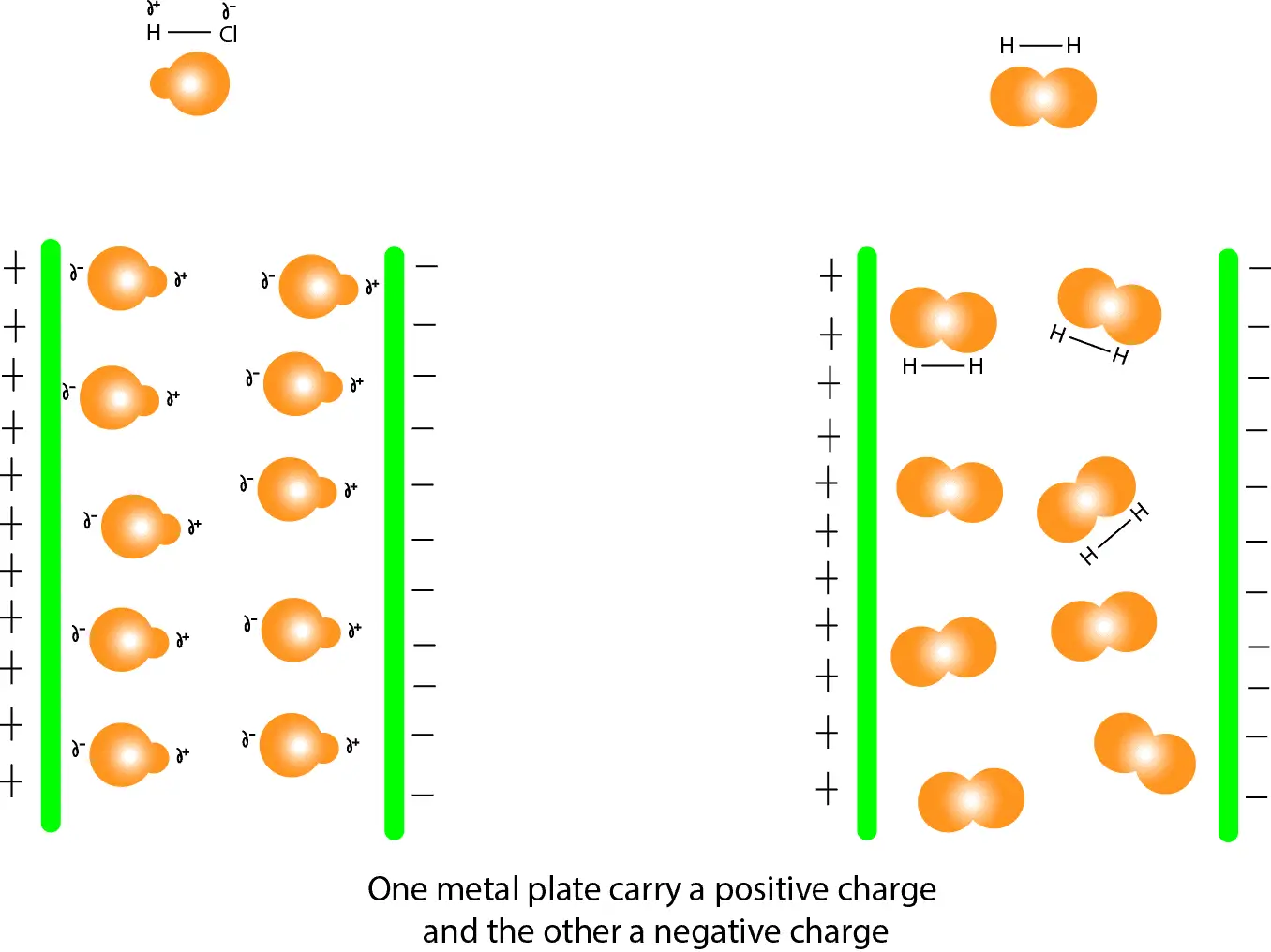
Why are some bonds or molecules polar?
Some bonds or molecules are polar when electrons are shared unequally. This means that the bonding electrons usually spend more time around the atom that attracts the shared electrons the strongest (most electronegative atom) than they do around the atom that attracts the shared electrons the weakest (least electronegative atom). As a result, the most electronegative atom acquires a partial negative charge (delta, δ–). While the least electronegative element acquires a partial positive charge (delta, δ+). When one end of a molecule carries a positive charge and the other end a negative charge, we usually say that the molecule is polar.
Let’s use the following models to illustrate the difference.
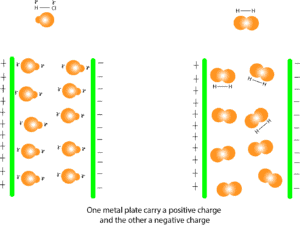
From the model, you can see that HCl is affected by the two charged plates. That’s the partial positive end of all the HCl molecules consistently point to the negatively charged end of the plate, and their partial negative end consistently point to the positively charged end of the plate. This arrangement confirms that HCl has a polar bond, therefore it’s polar. For the H2 molecule, you can see that the charged plates have no effect on it. This confirms that that H2 is nonpolar.
From this, we can say that a polar bond has a dipole moment in which its two electrical poles (one negative and the other positive) do not coincide but are separated by a finite distance. This dipole moment is usually represented by a dipole moment vector as shown in the following molecules.
Can molecules be nonpolar even though they contain polar bonds?
Yes, they can. Let’s consider these two simple molecules below.
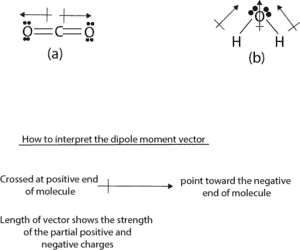
From figure (a), the two dipole moment vectors are pointing in opposite directions and because CO2 is linear, the dipole vectors cancel each other out, resulting in a net dipole moment of zero.
From figure (b), the two dipole moment vectors are pointing in the same direction, and because water has a bent shape, the two dipole vectors add up to get a net dipole moment that’s non-zero. Because of this, water is a polar molecule. If water molecule had been linear, its dipole moment would have been zero. Therefore, the shape of a molecule is critical in determining its dipole moment.
Why’s it that water molecules stick together?
Water molecules can stick together because the partially positive end of one molecule usually attracts the partially negative end of the other. This intermolecular force between polar molecules are usually called dipole-dipole forces. Let’s use the model below to illustrate dipole-dipole forces between water molecules.
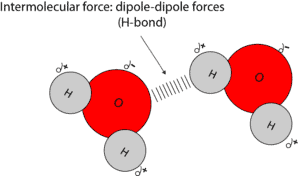
From the model, the dashes represent the invisible dipole-dipole forces holding two water molecules together. Although these forces are not as strong as covalent or ionic bond, it’s sometimes called the hydrogen bond. A hydrogen bond is formed when a hydrogen atom is directly bonded to an atom with a higher electronegativity. Examples of atoms with much higher electronegativity include, O, N, and F.