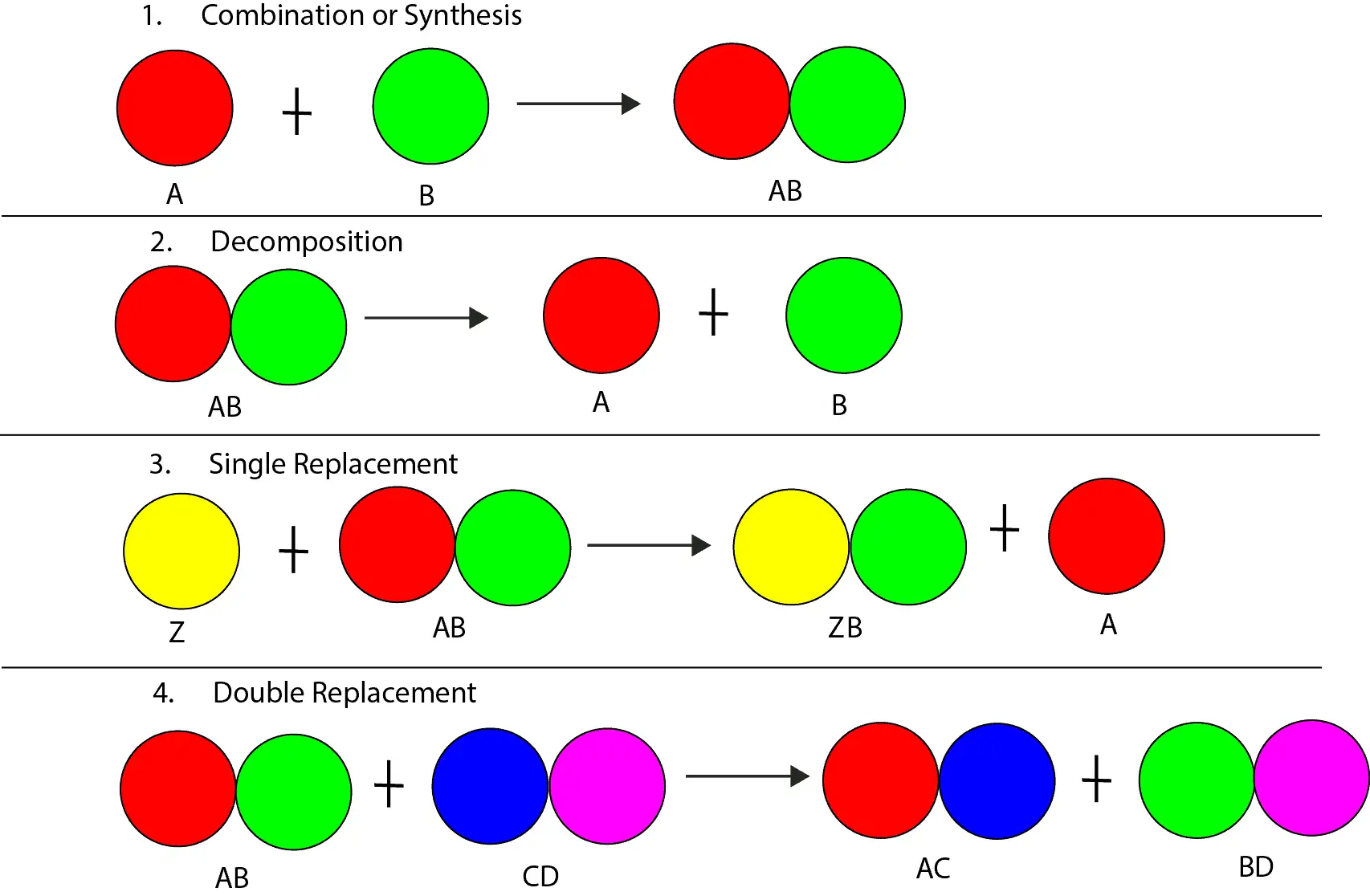
Is a chemical reaction the same as a chemical change?
A chemical reaction is the same as a chemical change. And a chemical reaction occurs when two or more chemicals react to produce a new chemical with unique physical and chemical properties. The chemicals that react to produce the new chemicals are usually called the reactants. The reactants usually consist of atoms put together in a specific way. The new chemicals that appear when the atoms in the reactants regroup are usually called products. The products usually consist of the same atoms in the reactants put together in a new way. Let’s use the following models to illustrate the concept.
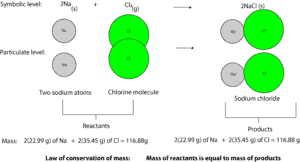
From the model, sodium (Na) combines with chlorine (Cl) to make sodium chloride (NaCl). Chlorine is a toxic choking greenish gas, while sodium is a soft metal that reacts explosively with water. And sodium chloride is a whitish salt vital for our survival.
Signs that a chemical reaction has occurred
Here are some of the signs of a chemical change when two or more chemicals combine:
- A new substance, which may be liquid, solid, or gas, appears.
- Energy is released to the surroundings as light
- Energy is released to the surroundings as heat.
- Energy is absorbed from the surroundings.
- A new color different from that of the reactants appears.
Do atoms gain or lose mass when they react with each other?
No mass is lost or gained when atoms combine with each other during a chemical reaction. As a result, the mass of atoms before reaction is always equal to the mass of atoms after reaction. This statement is referred as the law of conservation of mass. And it’s summarized as the mass of reactants is always equal to the mass of the products.
Will the mass change when a gas appears as a product in a chemical reaction?
If the gas is contained and not allowed to escape during a chemical reaction, then the mass of the reactants will be equal to the mass of the products.
Is energy always released or absorbed during a chemical reaction?
Depending on the type of reaction, energy can be released or absorbed from the surroundings. For molecules to react with each other, energy is always required to pull the atoms apart (break bonds) so that they can combine with other atoms. When these atoms eventually combine (bond) energy is always released to the surroundings. Whether energy is released or absorbed from the surroundings during a chemical reaction depends on the energy difference between two processes—bond breaking and bond making. When the energy needed to break chemical bonds is smaller than the energy released when atoms combine (bond making), the excess energy is released to the surroundings as heat. Conversely, when the energy needed to break bonds is larger than the energy released, energy is usually absorbed from the surroundings.
Why do some chemical bonds need more energy to break?
It all depends on the strength of the chemical bond. Some atoms hold each other much more strongly. As a result, molecules that have stronger bonds tend to be more stable, and so need more energy to break. They also tend to have lower potential energy. Potential energy is related to how atoms arrange with one another and the force fields surrounding them. If the atoms arrange favorably with one another, their potential energy usually decrease, and this decrease usually make the molecule more stable.
To summarize, multiple covalent bonds are stronger than single covalent bonds.
Energy is ALWAYS PUT IN to break a bond, WHILE energy is ALWAYS RELEASE when atoms make a bond.
Can atoms or molecules break their own chemical bonds?
Molecules or atoms can break their own bonds when they collide with one another. At the molecular level, the molecules of the solid metal chair you are currently sitting on are constantly vibrating (moving). Because they are constantly moving, we usually say that they possess kinetic energy. We can increase this kinetic energy if we heat the chair in a flame.
Similarly, the molecules of hydrogen and oxygen gas are constantly moving. If we combine a certain amount of oxygen with a certain amount of hydrogen in a closed container, the gas molecules will constantly collide with one another and the walls of the container. As they collide, these gas molecules will absorb some of the energy from the impact, and this energy can begin to break the covalent bonds within the gas molecules.
As an analogy, if a speeding car collides with another speeding car, the kinetic energy resulting from the impact usually cause the cars windows to break, tires to explode, and metals to crumple. However, car crashes differ significantly from molecular collisions. In molecular collisions, the atoms immediately regroup to form new covalent bonds. For instance, when the covalent bonds within the oxygen and hydrogen molecules break as a result of a collision, they immediately combine with each other in a ratio of two atoms of hydrogen to one atom of oxygen to make water.
What’s an endothermic reaction?
A reaction that absorbs energy from the surroundings. An example is a cold pack. At the macroscopic level, we usually know a reaction is endothermic when the reaction vessel in which the reaction runs feels cool when we touch it.
What’s an exothermic reaction?
A reaction that releases energy to the surroundings. A burning candle is an example of an exothermic reaction (combustion). At the macroscopic level, we usually know a reaction is exothermic when the reaction vessel in which the reaction runs feels warm when we touch it.
To read more about the types of chemical reactions, click here.