How to prepare a chemical solution from scratch
To prepare a chemical solution, you will need at least two things:
- Solute
- Solvent
The solute is the chemical in lesser amount, while the solvent is a chemical in larger amount. The solute usually dissolves in the solvent. The solute or solvent can be solid, liquid, or gas.
For example, to prepare sodium chloride (NaCl) solution from scratch, you must first decide on the concentration of sodium chloride you want. Since we will dissolve our NaCl in water, we will call water our solvent and NaCl our solute.
For example,
To prepare 500 mL of 5.0 M NaCl solution from scratch, you will
need to do the following:
- calculate the amount of NaCl you will need to weigh
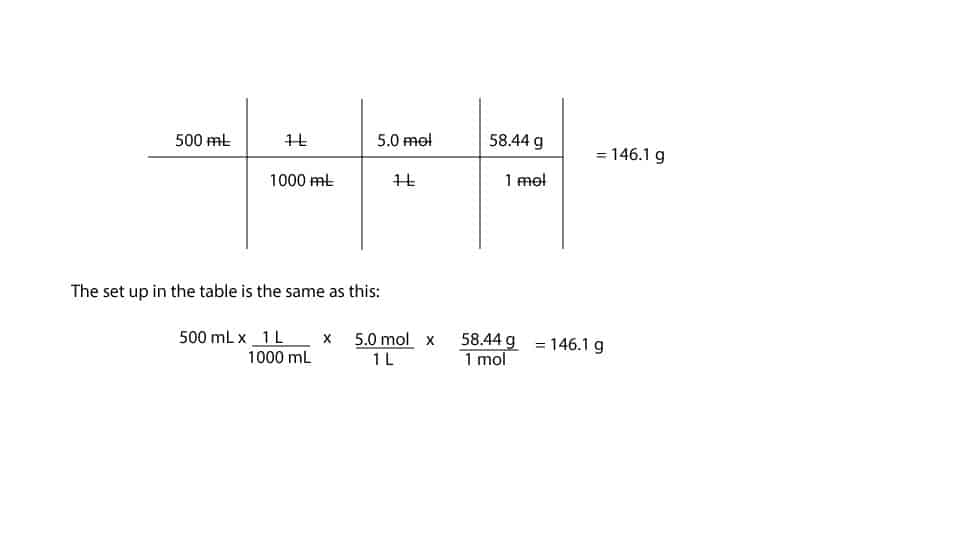
In order to calculate the amount of NaCl you will need to weigh, you must first convert the 500 mL to Liters, then to moles, and then to grams.
- To go from mL to liters, you will need the conversion factor (1L/1000mL), which is derived from the relationship 1000 mL = 1L.
- To go from liters to moles, you will need the conversion factor (5 mol/L), which is the same as the concentration of the solution you are preparing: 5M (5 mol/L).
- To go from moles to grams, you will need the conversion factor (58.44 g/mol), which is the same as the molar mass of the chemical you are using to prepare the solution. In this example, the chemical is: NaCl.
- Below, see the full calculation of the amount of NaCl you need to weigh to prepare your 500 mL of 5 M concentration of NaCl.
Answer
The 5.0 M NaCl solution means that we have 5.0 mol of NaCl present in 1 liter solution of NaCl.
Since Molarity is reported in moles per liter, we must convert the 500 mL to Liters (L). Since 1000 mL = 1L, we can set it up such that the milliliters (mL) cancel each other out, the liters (L) cancel each other out, and lastly, the moles cancel each other out. If we do, the overall set up will appear like so:

To prepare the 500.0 mL of 5.0 M NaCl solution,
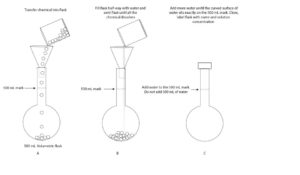
- weigh 146.1 g of NaCl into a volumetric flask.
- Add enough water and swirl until all the NaCl dissolves.
- Top it up with more water to the 500 mL mark on the volumetric flask.
After following through, you will realize that you can use the above mathematical expression to calculate the amount of any chemical you need to weigh to prepare a chemical solution.
After your calculation, here is an illustration showing how you can use a volumetric flask to prepare a chemical solution of known concentration, mass, and volume:
What’s a solution?
A solution is a uniform (homogeneous) mixture of solute and solvent. A solution can exist as gas, liquid, or solid.
To learn how to calculate solution concentration, click here, and how to prepare solution from stock, click here.