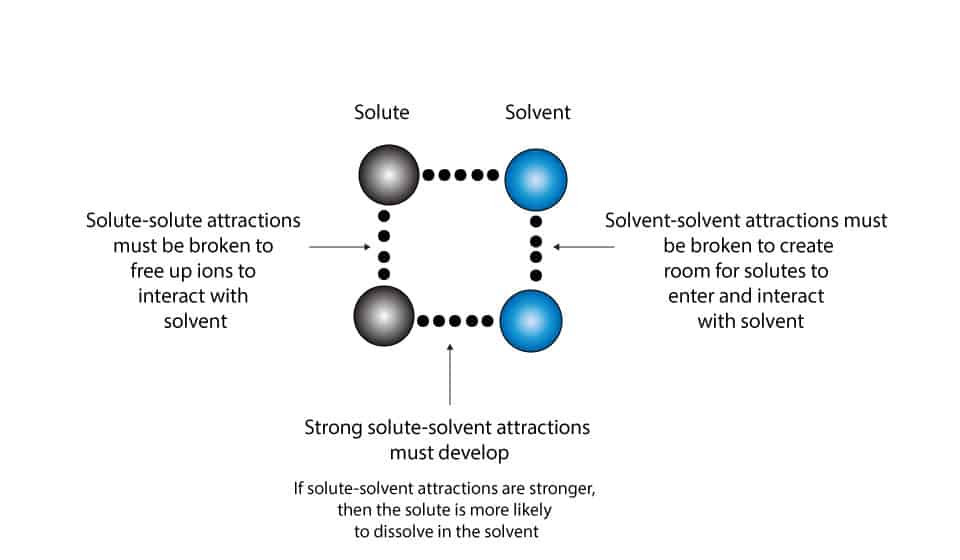
Why does one chemical dissolve in another?
For one chemical to dissolve in another, these three steps must happen at the same time during the dissolving process. The steps are:
Ist step:
- the ions or molecules of solute must break the attractive forces between them so that they can free up themselves to interact with the solvent.
2nd step:
- the ions or molecules of solvent must break the attractive forces between them so that they can make room to allow the solute ions or molecules to interact with the solvent.
3rd step:
- the ions or molecules of solute must strongly attract the ions or molecules of solvent.
Let’s use the following model to illustrate the three steps.
When will a solute dissolve in a solvent?
A solute will dissolve in a solvent when the 3rd step generates enough energy to drive the 1st and 2nd steps.
Why’s energy necessary to drive the 1st and 2nd steps?
To form a solution,
1st step:
- the ions or molecules of solute must absorb energy to break the attractive forces between them. As you can tell, the stronger the attractive forces, the more energy you will require to overcome these forces.
2nd step:
- the ions or molecules of solvent must absorb energy to break the attractive forces between them. Similarly, the stronger the attractive forces, the more energy you will require to overcome these forces.
3rd step:
- the ions or molecules of solute must strongly attract the ions or molecules of solvent. The stronger the attraction, the more energy is released into the dissolving process.
Now, if we represent the change in energy for the 1st step as ΔEstep1, 2nd step as ΔEstep2, and 3rd step as ΔEstep3, then we can say that the total energy change when a solute dissolve in a solvent is the sum of the three steps: ΔE total = ΔEstep 1 + ΔEstep 2 + ΔEstep 3.
Since step 1 and 2 absorb energy, we can say that the change in energy for these steps is positive. Since step 3 releases energy, we can say that the change in energy for step 3 is negative. Now, if the amount of energy released in step 3 is larger than the amount of energy absorbed in step 1 and 2, then the solute will dissolve in the solvent. This happens because step 3 generates enough surplus energy to drive step 1 and 2. Thus, chemists will say, if ΔE total < 0, the solute will dissolve in the solvent.
On the other hand, if the amount of energy released in step 3 is smaller than the amount of energy absorbed in step 1 and 2, then the solute will not dissolve. This happens because step 3 generates an energy loss. Thus, chemists will say, if ΔE total > 0, the solute may not dissolve in the solvent. May in the sense that some solutes dissolve in solvents when we heat the solute-solvent mixture.
General rule to determine whether a solute will dissolve in a solvent
The general rule is often summarized as “like dissolves like.” Like as used here means that:
- Polar solutes will dissolve best in polar solvents
- Non-polar solutes will dissolve best in non-polar solvents
This rule make sense because when the solute and solvent have similar polarities they are more likely to generate surplus energy to drive step 1 and 2 forward.
To learn how to calculate solution concentration, click here.
To learn how to prepare solutions, click here.
To learn about how sodium chloride dissolves in water, click here.