Properties of acids and bases
Properties of acids and bases can be described at the macroscopic and molecular (microscopic level).
At the macroscopic level, acids
- taste sour
- turns blue litmus paper red
- reacts with metals to produce hydrogen gas
- reacts with carbonates to produce carbon dioxide
- neutralize bases in an acid-base reaction to produce salt and water
At the macroscopic level, bases
- taste bitter
- produces aqueous solutions that feels slippery
- turns red litmus paper blue
- neutralize acids in an acid-base reaction to produce salt and water
At the molecular level,
- According to Arrhenius, an acid is a substance that dissociates in water to produce H+ These highly reactive H+ ions are easily picked up by water to form hydronium ions, H3O+. Here is an example of hydrochloric acid (HCl) dissociating in water: H-Cl(g) → H+(aq) + Cl–(aq). The hydrogen ion (H+) gets picked up by water to form hydronium ion: H+ + H2O → H3O+. Here is a model showing the hydrogen ion transfer:
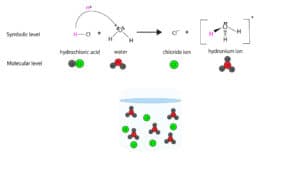
Dissociation of HCl in water Arrhenius definition excludes chemicals that do not produce hydrogen ions directly, but show acidic behavior. For this reason, chemists have another definition for an acid. And here is it:
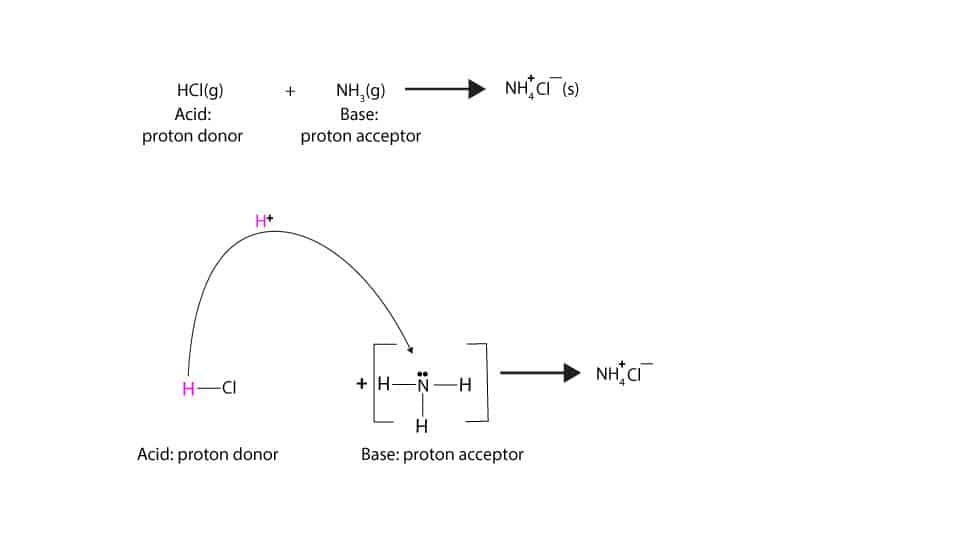
- According to Brφnsted-Lowry, an acid is any chemical that donates a proton.
H-Cl(g) + NH3(g) →NH4+Cl–(s) (proton donor: HCl; Proton acceptor: NH3,ammonia). The proton is the naked nucleus of the hydrogen atom that easily gets transferred in acid-base reactions. Here is a model showing proton transfer.
At the molecular level, bases
- According to Arrhenius, a base is a substance that dissociates in water to produce hydroxide ions, OH– Here is an example of sodium hydroxide(NaOH) dissociating in water to produce sodium ions (Na+) and hydroxide ions (OH–). NaOH(s) → Na+(aq) + OH–(aq). Arrhenius definition excludes chemicals that do not produce hydroxide ions directly, but show basic behavior. For this reason, chemists have another definition of a base. And here is it:
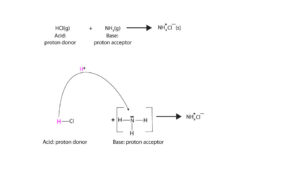
- According to Brφnsted-Lowry, a base is any chemical that accepts a proton.
H-Cl(g) + NH3(g) → NH4+Cl–(s) (proton donor, acid: HCl; Proton acceptor, base: NH3). Here is model showing Brφnsted-Lowry base.

To read about the difference between strong and weak acids, click here.