What’s atmospheric pressure?
Atmospheric pressure is pressure exerted by air molecules in the atmosphere as they constantly bounce off things. Pay attention to text in bold “ constantly bounce off things.” Air or gaseous molecules must bounce off or collide with the walls of the container they are in to exert pressure. Just imagine this; as you blow air in a balloon or pump air into a car or bike tire, the air molecules must collide with the balloon or tire wall to exert pressure and cause the balloon or tire to grow in size.
Pressure is defined here as the force gas molecules exert as they strike an area on a surface.
Mathematically, pressure can be expressed as P = F/A, where
F is force , which has units of Newton (N)
A is area, which has units of square meter (m2)
P is pressure, which has units of Newton per square meter (N/m2)
How can we measure atmospheric pressure?
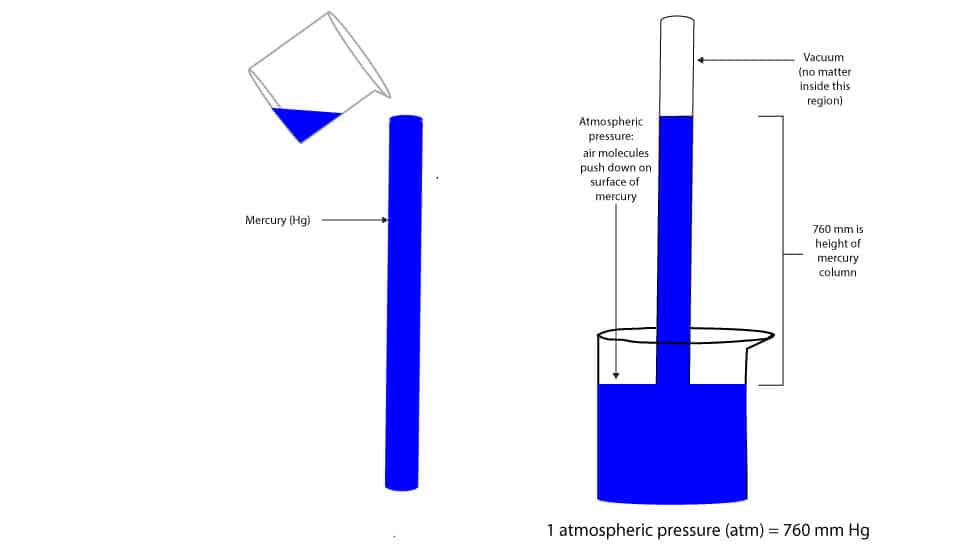
We can measure atmospheric pressure by a mercury barometer. A mercury barometer consists of a glass tube of at least 760 mm long with one end of the tube sealed and the other end opened. We can then fill this tube with mercury and invert the opened end into a beaker of mercury. The first barometer designed by an Italian scientist called Evangelista Torricelli, is shown below.
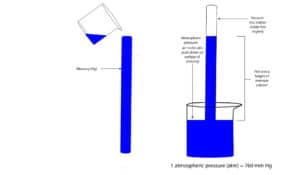
As you can see, at sea level, the mercury level dropped to a height of 760 mm, leaving no air above the mercury level in the tube.
Why did mercury drop to the same level?
The atmospheric pressure acting downwards on surface of mercury in beaker stops the mercury from draining out. This balance of forces is achieved when the weight of mercury in tube is equal to the air pressure pushing downward on surface of mercury in beaker. This pressure at sea level which supports a column of mercury at a height of 760 mm is called the standard pressure. This standard pressure can be expressed in many units. This units include:
- mm of mercury (760 mm of mercury)
- atmosphere (atm), 1.00 atm
- torr (1 torr = 1 mm of mercury), 760 torr
- pounds per square inch (psi or lb/in2) = 14.7 lb/in2 or 14.7 psi
- inches of mercury, 29.9 inces of mercury
- pascal (Pa), 101,325 Pa
From these units, we can write relationships such as:
1 atm = 760 mmHg = 14.7 lb/in2 or 1 atm = 760 torr = 14.7 lb/in2
As you can tell, we can use these relationships to set up conversion factors to convert from one unit of measurement to another. To read more about conversion factors, click here.
Can we use other liquids like water other than mercury?
Yes, we can. However, it’s more practical to use mercury. This is because as the density of mercury is greater than that of water, the atmospheric pressure will push mercury up the tube much less than it will push water. Thus, with mercury, we get a shorter mercury column height, while with water, we get a taller water column height.
What factors can affect atmospheric pressure?
- Altitude
- Weather
How does altitude affect atmospheric pressure?
As altitude increase, atmospheric pressure decrease. As you go higher and higher in the atmosphere, there are less and less air molecules.
How does weather affect atmospheric pressure?
During winter, as air molecules over land cool, they move much more slowly. This loss of kinetic energy causes the air molecules to move closer to one another and become denser than the surrounding air. This increase in density cause the air molecules to sink, increasing atmospheric pressure. During summer, as air molecules over land warm up, they move much faster. This gain of kinetic energy causes the air molecules to spread out more than the surrounding air. This decrease in density cause the air molecules to rise, decreasing atmospheric pressure.
To learn more about general properties of gases, click here.