What’s the relationship between pressure and amount of gas?
The pressure (P) of gas is directly proportional to the amount of gas when we hold temperature and volume of gas constant. This means that the pressure of gas will increase as the amount of gas molecules increase inside a container with a fixed volume and at a fixed temperature.
Mathematical, we can express this relationship as: P ∞ n.
If we remove the proportionality sign and introduce an equal sign and a proportionality constant, we will get this: P = Kn
If we divide both sides of the equation by n, we will get this
P/n = K.
Now, let’s use the following model to illustrate the relationship.
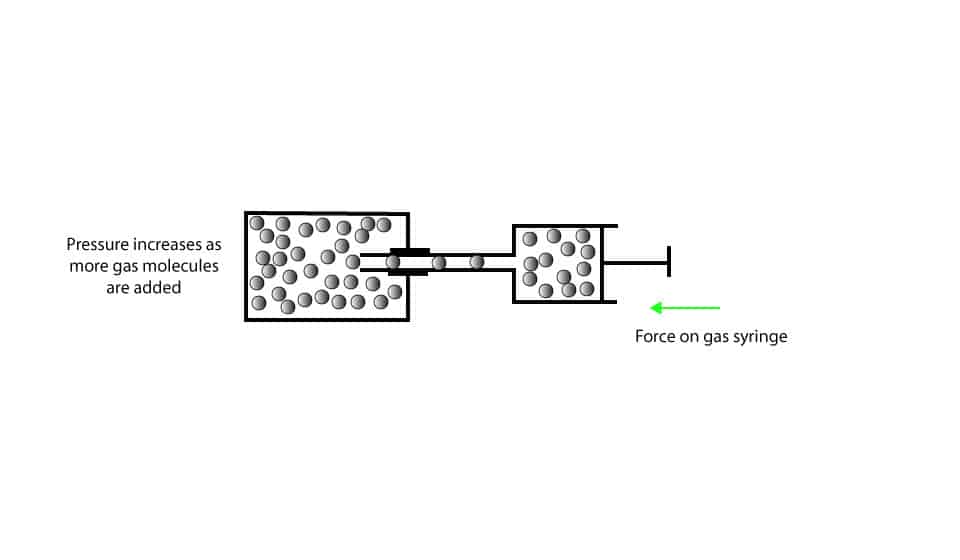
As you can see, the metal cylinder has a fixed volume. When we slowly push the gas syringe, gas molecules are delivered into the cylinder. As we continue to do this, we deliver lots of gas molecules into the cylinder, increasing the gas pressure inside the cylinder.
Why does pressure increase when we add more gas molecules?
When we inject more gas molecules into the cylinder, more gas molecules collide with the walls of their container, increasing the pressure. In the same way, when we inject fewer gas molecules into the cylinder, fewer gas molecules collide with the walls of the cylinder, decreasing the pressure.
To read about factors that can affect gas behavior, click here.