What’s the relationship between volume and amount of gas?
The volume (V) of gas is directly proportional to the amount of gas when we hold temperature and pressure constant. This means that at fixed temperature and fixed pressure, the volume of gas will increase as the amount of gas molecules increase inside a metal cylinder with a movable piston.
If you imagine blowing air into a balloon, you will realize that as you blow more air molecules into it, the balloon grows in size, increasing the amount of space the air molecules takes up in the balloon.
How can we keep the pressure constant inside the cylinder?
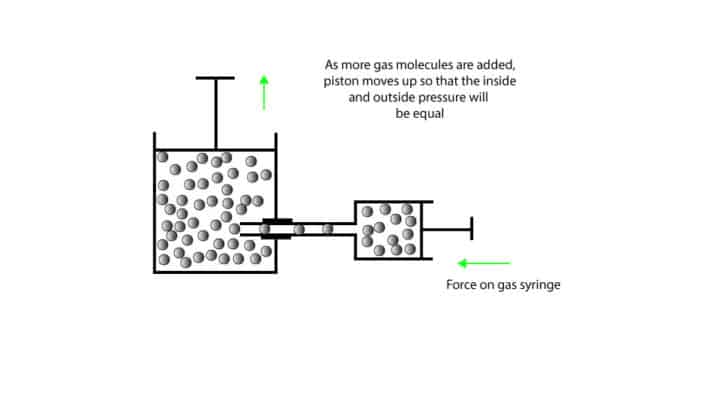
We keep the gas pressure inside the cylinder constant by fitting it with a movable piston that can either go up or down depending on the pressure inside the cylinder. Since the pressure outside the flask stays unchanged, we say that the outside pressure (atmospheric pressure) is constant. Which means that if the pressure inside the cylinder is greater than the atmospheric pressure, the piston will move up until such a point that the inside pressure equals the outside pressure. If the pressure inside the cylinder is smaller than the atmospheric pressure, the piston will move down.
Can we mathematically express the relationship between volume and amount?
Yes, we can. Mathematical, we can express this relationship as: V ∞ n.
If we remove the proportionality sign and introduce an equal sign and a proportionality constant, we will get something like this: V = Kn
If we divide both sides of the equation by n, we will get this
V/n = K.
This volume-amount relationship is usually called Avogadro’s law in honor of Avogadro, who was first to uncover the relationship
If you plot a graph of volume versus moles of gas, you will get a graph like this:

Now, let’s use the cylinder-piston model to illustrate the relationship.
Since the piston can move up or down, we say that the gas inside the cylinder has no fixed volume. When we slowly push the handle on the gas syringe, gas molecules are delivered into the cylinder. As we continue to add more and more gas molecules the pressure inside the cylinder builds up. To ease this pressure, the metal piston moves up until such a point that the pressure inside the cylinder equals the atmospheric pressure.
Why does volume increase when we add more gas molecules?
As more and more gas molecules collide with the walls of their container, they build pressure inside the container. To reduce this pressure, the volume of gas must increase. Once the gas volume increase, gas molecules must travel longer distances to strike the walls of their container. As a result, these molecules will strike the container less often, reducing the pressure inside the cylinder.
To learn about the factors that affect gas behavior, click here.