When ice (water) is heated, does its temperature rise continuously?
No! The temperature of ice or water does NOT rise continuously when its heated. Let’s use the graph below to explain.
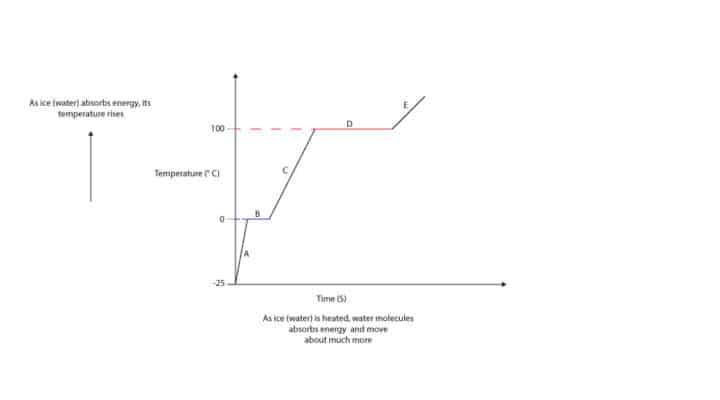
From the graph, when ice at -25 °C is heated, its temperature starts to rise (A) until its temperature reaches 0 °C (B), where the temperature stays constant. At 0 °C, the ice melts and turns into liquid water. When all the ice is completely turned into liquid water, its temperature starts to rise again (C) until it reaches 100 °C, where the temperature stays constant. At 100 °C, the liquid water boils and turns into steam (D). When all the liquid water is completely turned into steam, its temperature starts to rise again (E).
Why does temperature of water increase when heated?
When water is heated on a stove, energy is absorbed by water molecules. As these molecules absorb energy, they move about much more, increasing their kinetic energy. This increase in kinetic energy cause the temperature of water to rise. So,
What’s temperature?
Temperature is a measure of how intense the average kinetic energy is in a substance. Why average? Molecules do not move at the same speed, as a result they have different kinetic energy. Molecules that move faster have higher kinetic energy than molecules that move slower. So, temperature is a measure of how intense the average kinetic energy is. This means that a warmer material has a higher temperature and kinetic energy than a colder material.
Why does temperature of water stay constant at 0 °C and 100 °C
The temperature stays constant at 0 °C and 100 °C because the energy the molecules absorb at these points is used to break the attractive forces between them.
Why break the attractive forces between molecules?
It’s necessary so that the ice can transition to water and steam. We all know that, at 0 °C ice turns into liquid water (phase change). But keep in mind that for ice to turn to liquid water, the water molecules in ice must break the attractive forces between them such that they can slide over one another. Similarly, for liquid water to turn to steam at 100 °C, the water molecules must break the attractive forces between them such that they can move at random to fill any container.
How does kinetic and potential energy vary when ice is heated?
When ice is heated, the kinetic energy of water molecules rises. Kinetic energy rises because water molecules absorb energy and begin to move more. From the graph, regions of rising kinetic energy are: A, C, and E. However, while the ice is being heated, its temperature stops rising at 0 °C. And at 0 °C, the kinetic energy remains constants while the potential energy increases.
Why does the temperature stop rising at the melting point of ice?
It stops rising because the energy that’s absorbed is used to pull the molecules apart so that they can transition from ice to liquid water. This “pulling apart” means to break the attractive forces between water molecules so that they can slide over each other to form the liquid state. Because the temperature stays constant at the melting point, the kinetic energy also stays constant. But
Will potential energy of water molecules increase at the melting and boiling points?
Yes, potential energy of water molecules will increase, while kinetic energy stays constant. Potential energy grows because the distance between water molecules increase. And keep in mind that the energy that’s put in to keep the molecules away from each other so that they can exist as liquid or gas is what we call potential energy. We can get back this energy when we cool steam (gas) back to water (liquid) or water back to ice (solid).
What phase changes occur when ice is heated and are these phase changes physical changes?
These phase changes are physical changes, and they are:
- Melting at 0 °C, when ice turns into liquid water
- Boiling at 100 °C, when liquid water turns into steam (gas)
From the graph (heating curve of water), you can see that the region at which ice melts is shorter than the region at which water boils. This means that it takes much more energy to boil water than it takes to melt ice.
To learn how temperature and kinetic energy vary as steam cools, click here.
To learn more about the cooling curve of water click here.