When steam (water vapor) cools, does its temperature drop continuously?
No! The temperature of steam or water does NOT drop continuously when it cools. Let’s use the graph below to explain.
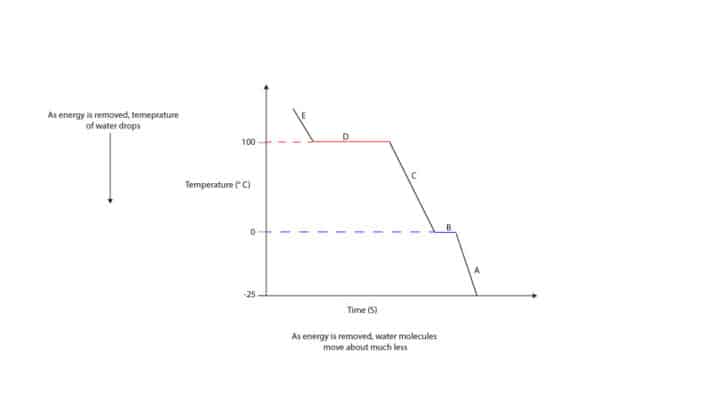
From the graph, when steam at 125°C cools, its temperature starts to drop (E) until it reaches 100 °C (D). At 100 °C, the steam condenses and turns into liquid water. When all the steam is completely turned into liquid water, its temperature starts to drop again (C) until it reaches 0 °C. At 0 °C, the liquid water freezes and turns into ice (B). When all the liquid water is completely turned into ice, its temperature starts to drop again (A).
Why does steam (gas) form water (liquid) when it cools?
When steam cools, energy is removed from water molecules. As these molecules transfer their energy to the cooler surroundings, they move about much less, decreasing their kinetic energy. This decrease in kinetic energy cause water molecules to slightly stick together. Why would they stick together? They do because water molecules are polar. As a result of this polarity, water molecules stick together to form water when their kinetic energy significantly decrease.
How does kinetic energy vary when steam cools?
When steam cools, the kinetic energy of water molecules drops and the molecules move much less. Kinetic energy drops because water molecules transfer their energy to the colder surroundings. From the graph, regions of decreasing kinetic energy are: E, C, and A. However, at condensation and freezing points the kinetic energy and temperature stays constant.
What phase changes occur when steam cools and are these changes physical changes?
These phase changes are physical changes, and they are:
- Condensation at 100 °C, when water vapor (gas) turns into water (liquid water)
- Freezing at 0 °C, when liquid water turns into ice (solid water)
From the graph (cooling curve of water), you can see that the region at which water vapor condenses is longer than the region at which liquid water freezes. This means that much energy must be removed from steam to turn it into liquid water. From the graph, you can also see that
- water boils (boiling point) and condenses (condensation) at the same temperature
- melts (melting point) and freezes (freezing point) at the same temperature
Is energy conserved when water changes phases?
Yes, energy is conserved. The amount of energy we use to heat ice to turn to steam is the same amount of energy we get back when we cool that same steam back to ice. Thus, we say that energy is conserved.
To learn more about the cooling curve of water, click here.
To learn how temperature and kinetic energy vary as ice turns into steam, click here.