Can atoms share electrons unequally in a covalent bond?
Yes, they can.
Why do atoms share electrons unequally in a covalent bond?
When atoms differ in electronegativity, the most electronegative atom will draw the shared electrons more strongly to itself. When this happens, we usually say that the electrons are shared unequally between the atoms.
What is electronegativity?
Electronegativity (EN) is a measure of an atom’s ability to attract shared electrons in a covalent bond to itself. Here is a table showing the electronegativity values of the main group elements.
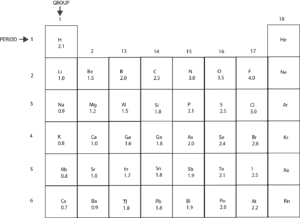
From the table, we can construct an electronegativity scale starting from the lowest value of 0.7 to the highest value of 4. The scale will appear like this:

Based on the scale, Cesium (Cs) is the least electronegative element because it’s unable to strongly attract shared electrons to itself. Fluorine (F) is the most electronegative because it can strongly attract shared electrons to itself.
Notice that metals generally have lower electronegativity values, while nonmetals have higher electronegativity values.
In addition, elements in group 18 are generally stable and unreactive under normal conditions, as a result, they have no electronegativity values.
How to predict whether atoms will react to form covalent or ionic bond
We can use these electronegativity values to predict whether atoms will react to form covalent or ionic bond. Thus,
- when atoms have similar electronegativity values, they are more likely to share than to transfer electrons to each other.
- when atoms have different electronegativity values, the atom with the lower value is more likely to transfer an electron to the atom with the higher value.
We can also use the electronegativity values to construct a scale. This scale will be called the electronegativity difference (DEN) scale. And it will start from 0.0 for elements with identical electronegativity values to 4.0 for elements with higher electronegativity.
On the scale, how can we decide on a cut-off point below which the bond will be covalent, but above which the bond will be ionic?
It’s not clear-cut. In nature there is a gradual change in covalent bond as we move from pure covalent on the left side of the scale to ionic on the right side of the scale. Nevertheless, we know that the bond in sodium chloride (NaCl) is ionic, and the electronegativity difference between chlorine and sodium is: 2.1 (3.0 – 0.9). If we set the cut-off point at 2.0, the scale will appear like this:
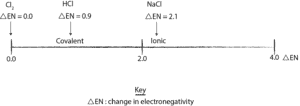
From the scale, we can deduce the following:
- a change in electronegativity greater than 2.0 means that the bond is ionic
- a value of less than 2.0 means that the bond is covalent.
However,
When a bond is covalent, it can either be pure covalent or polar covalent.
- when it’s pure covalent, it usually means that the electrons are shared equally
- when it’s polar covalent, it usually means that the electrons are shared unequally
And when the electrons are shared unequally, the bonding electrons usually spend more time on one atom than on the other. And when this happens, it usually generates a partial negative (delta, δ–) charge on the most electronegative atom and a partial positive charge (delta, δ+) on the least electronegative atom. Let’s use the following model to further show this difference.
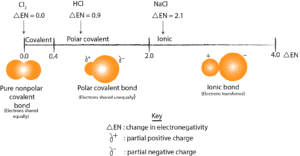
From the diagram, chlorine molecule (Cl2) is pure covalent because the two chlorine atoms share the electrons equally. However, HCl is polar covalent because the hydrogen and chlorine atoms share the electrons unequally, with the bonding electrons spending more time with the most electronegative element, Cl. As a result, the hydrogen end has a partial positive charge, while the chlorine end has partial negative charge. When HCl is placed between two charged plates the positive end of the molecule will be attracted to the negative pole of the plate, while the negative end of the molecule will be attracted to the positive pole of the plate.