How hydrogen atoms share valence electrons to form covalent bond and hydrogen molecule (H2)
First, if we write the electron configuration of hydrogen, you will realize that hydrogen has only one valence electron in the one-s orbital (1s1). As a result, the hydrogen atom is unstable. Because it’s unstable, hydrogen will always bond with another atom so that it can become stable and achieve a stable electron configuration similar to that of helium (He): 1s2.
Where can hydrogen get this other electron?
One way hydrogen can get this other electron is to bond with another hydrogen atom. So, let’s imagine that we have two hydrogen atoms with their valence electrons depicted by these two circles.
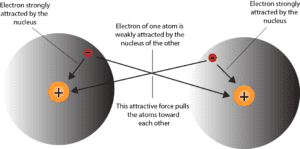
As you can tell, the orange circle with the plus sign describes the nucleus, which usually carries a positive charge because of the protons in it. While the red circle with the minus sign describes the electron, which usually carries a negative charge.
To keep the hydrogen atom intact, its nucleus must attract its electron (opposite attract). Now, when the two separate hydrogen atoms are far apart, there is virtually no interaction between the two atoms. However, as the two hydrogen atoms gain energy and move toward each other, they reach a certain distance where the two hydrogen atoms feel the presence of each other.
That is the nucleus of the hydrogen atom on the left will attract the electron of the hydrogen atom on the right. Likewise, the nucleus of the hydrogen atom on the right will attract the electron of the hydrogen atom on the left. As the attractive force between the two atoms strengthens, the atoms pick up more speed and continue to move closer to each other. However, if these atoms suddenly become too close, they will immediately repel each other.
Why will the hydrogen atoms repel each other?
If they are too close, the nucleus of the left atom will repel the nucleus of the right atom. Likewise, the electron of the left atom will repel the electron of the right atom (like charges repel).
When’s the covalent bond formed between the two hydrogen atoms?
When the attractive and repulsive forces between the two hydrogen atoms is just right, they will form the covalent bond. Here is a model to help you visualize its creation.
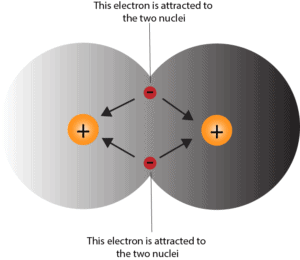
Notice, because the electrons are equally attracted to the nuclei of both atoms, they are located between the two atoms. And these electrons hover and spend most of their time in the region once the covalent bond is created. The more covalent bonds atoms make, the stronger the force of attraction between them.
It’s tiring to keep drawing circles and pluses and minuses to explain covalent bonding. As a result, chemists have a shorthand for doing this. If we draw a line to connect the two dots depicting the two electrons shared by the hydrogen atoms, we will get something like a dash (__). The dash is a symbol chemist use to denote a covalent bond formed between two atoms.
If we have three of these dashes between atoms, it follows that we have three pair of electrons (6 electrons) shared between them. So, we can depict the hydrogen molecule as a structural formula like this: H__H (read as single bond between hydrogen atoms). But it’s not always necessary to show the dash anytime we write the chemical formula for a molecule. As a result, chemists sometimes condense the H__H to H2.
If you want to learn more about chemical bonding, click here.