What’s energy?
Energy is an abstract core concept that is widely discussed in science. You will find it in chemistry, physics, biology and other areas of science. Because of this, it’s sometimes called a crosscutting concept on which other concepts are built.
Energy is defined as the ability to do work. Although we can’t define energy like we did for an atom, we do know what it does. And can track its flow as it moves through matter. As a result, this definition is usually called an operational definition of energy.
In the past, Lavoisier, a prominent scientist, had thought energy, previously called caloric was a material and weightless-fluid that flows through matter, but that view is no longer true.
Yet, to make the concept of energy easy to understand, we will use the word “flow.” But, do not assume that “flow” as used here means energy is a fluid. We stress that Energy is not matter!
Why’s energy defined this way?
It’s defined this way because no one truly understands exactly what energy is. Yet, we do know it gives us the ability to do work. We also know we must burn gasoline or run batteries in our cars to drive them. With all this, whether we can see energy or not, we can deduce that energy is a property of matter that enables matter to do work.
What’re the effects of energy on the properties of matter?
Properties of matter change when its energy or temperature changes. These properties include pressure, density, electrical conductivity, hardness, and many others.
In fact, the flow and transformation of energy is what makes everything in the universe go. Without energy, the universe in itself dies.
What’re the forms of energy?
Energy can exist in two main forms:
- Kinetic energy
- Potential energy
Of these two forms, energy in itself remains unchanged as it transforms from one form to another. And the total energy in a substance is usually equal to the sum of kinetic plus potential energy.
Let’s use the following model to further illustrate the different forms of energy.
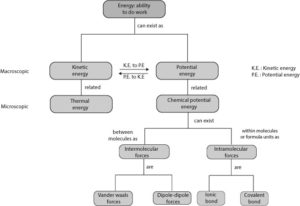
From figure 1, energy can exist as kinetic and potential energy.
At the macroscopic level:
- Kinetic energy is the energy something has because of its motion. Thus, kinetic energy is the energy associated with motion. For example, a moving ball or car has kinetic energy. And this kinetic energy can be calculated using a mathematical formula.
- Potential energy is the energy something has because of its position relative to another or how its parts are arranged relative to one another. For example, an apple hanging up on a tree has potential energy due to the earth’s gravitational pull on it. And this potential energy can also be calculated using a mathematical formula.
At the molecular level:
- Kinetic energy is related to thermal energy. And thermal energy is associated with temperature. When tiny particles such as electrons, atoms, molecules or ions move at random, they generate energy and heat called thermal energy.
- Potential energy is related to chemical potential energy. When electrons and nuclei of atoms arrange to make a bond, we call the energy associated with the bond chemical potential energy. Chemical potential energy can exist as intermolecular and intramolecular forces. Intermolecular forces are Vander Waals and dipole-dipole forces, while intramolecular forces are ionic and covalent bonds.
From figure 1, the double arrows pointing in opposite directions shows that kinetic energy can be converted to potential energy and potential energy can be converted back to kinetic energy. For example, the potential energy of an apple will immediately transform to kinetic energy once the apple is cut loose from the apple tree. Similarly, the potential energy that results when two or more atoms bond will immediately transform to kinetic energy once the atoms separate and begin to move away from one another.
What’re the sources of energy?
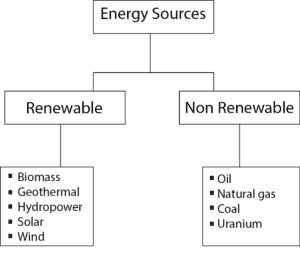
Sources of energy can be classified as:
- renewable and non renewable
Renewable energy sources
As long as the universe exist, renewable energy sources can be renewed or replaced by natural or ecological cycles.
Non renewable energy sources
Non renewable energy sources can’t be renewed in our life time. Technically, it takes millions of years to renew these sources.
Here is a diagram showing renewable and non renewable energy sources.
What’re the units of energy?
We can measure energy in:
- calorie, cal (read small calorie, starting with lower case c)
- Calorie, Cal (read large calorie, starting with upper case C) or kilocalorie, kcal. Usually called nutritional calories and commonly reported on the labels of our snacks.
- joules, J
- kilowatt-hour, kWh (units on our electric bills)
How are these units related to one another?
1 cal = 4.18 J
1 Cal = 1000 cal = 4180 J
1 kWh = 3.60 x 106 J
To convert from one unit to another, these are the conversion factors you will need.
To learn more about unit conversions, click here.
To learn more about the law of conservation of energy, click here.
