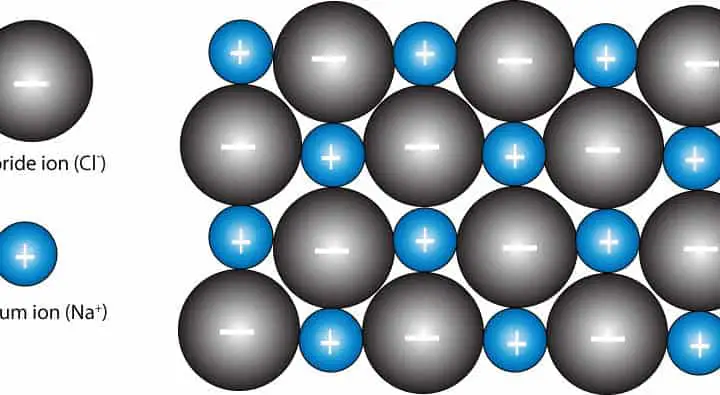
How are the ions in sodium chloride (NaCl) crystal arranged?
Solid sodium chloride (NaCl) crystals consist of sodium ions (Na+) and chloride ions (Cl–) held together in a repeating three-dimensional pattern called a lattice. In a three-dimensional view, each sodium ion is surrounded by six chloride ions and each chloride ion is surrounded by six sodium ions. As a result of this arrangement, the number six is usually referred as the coordination number of sodium and chloride ions. Here is a two-dimensional view of NaCl crystal lattice:
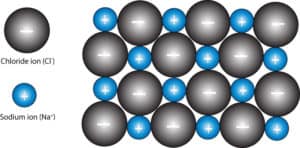
From the two-dimensional view, is misleading to think that each sodium ion is surrounded by four chloride ions and each chloride ion is surrounded by four sodium ions. If you consider the two-dimensional view to be in the plane of the paper, then in the three-dimensional view, you will have another ion pointing outward towards you, and another pointing backward away from you. If you add the imaginarily two ions to the four ions surrounding each ion, you will get six.
From the plus and minus signs, you can tell that the lattice is held together by strong electrostatic force called the ionic bond. Because of these strong bonds, sodium chloride must be heated to a temperature of 801 °C before it can melt into sodium and chloride ions. Yet, at room temperature this compound easily dissolves in water to generate mobile sodium and chloride ions.
Why is it that NaCl easily dissolves in water? Click here to learn more.