How do you calculate atomic mass?
To calculate the atomic mass of an element, we have to calculate how much each isotope contributes to the mass of the atom. To accomplish this, we usually use an approach called the weighted average. The weighted average takes into account the mass and percentage abundance of each isotope.
What’s percentage abundance?
It is the proportion of atoms of an isotope in a sample of an element taken from the natural world. Percentage abundance is always reported as a percentage, and it is calculated as: (number of atoms of an isotope) divided by (the total number of atoms of all isotopes of that element) multiplied by 100. Percentage abundance usually can be divided by 100 to get fractional abundance.
How do you use weighted average to calculate atomic mass?
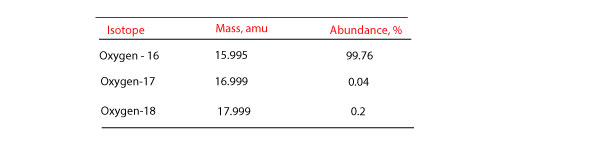
To use weighted average, we must take into account the mass and percentage abundance of each isotope. Let’s use the data in the following table to show how weighted average is used to calculate the atomic mass of oxygen.
Solution
To calculate the atomic mass of oxygen using the data in the above table, we must first
- multiply the mass of each isotope by its corresponding natural abundance (percentage abundance). But, since the abundance is in %, you must also divide each abundance value by 100.
And second,
- Sum the result to get the atomic mass of the element
Thus,
Atomic mass of oxygen = 15.995 amu (99.76/100) + 16.999 amu (.04/100) + 17.999 amu (.2/100)
= 15.956612 amu + 0.0067996 amu + 0.035998 amu
= 15.9994096 amu
= 16.00 amu
Note that the abundance in percent always add up to 100 %. Meaning if the previous question had left out the percentage abundance value for oxygen-18, you could have gotten it by subtracting the sum of the percentage abundance for oxygen-16 and oxygen-17 from 100% to get the percentage abundance for oxygen-18.
That is: 100% – (99.76% + .04%) = 0.2% for oxygen-18
Generally, you can apply this approach to figure out missing percentage abundance when you know the percentage abundance values for all, but one isotope.
If you examine the atomic masses on the periodic table, you will notice that they are fractional. Why are they fractional? They are fractional because atoms exist as isotopes. And isotopes do not have the same mass and abundance in nature. As a result, to calculate the atomic mass of an element, we have to calculate how much each isotope contributes to the mass of the atom.
Why are there no units of grams attached to the atomic mass of oxygen?
There are no units attached to atomic masses because atomic masses are relative atomic masses. Relative in this sense means one thing is compared to another. So, relative atomic mass means the mass of one atom is compared to the mass of another atom.
The atom to which other atoms are compared to is usually called the standard. At present, an isotope of carbon called carbon-12 (C-12) is selected as the standard and assigned an atomic mass of exactly 12 amu, where amu stands for atomic mass units. Therefore, the mass of every other atom on the periodic table is determined by how light or heavy it is when compared to the mass of C-12.
What instrument is used to measure the relative masses of atoms?
At present, mass spectrometry is the technique used to measure the relative masses of atoms and their percentage abundance in nature. In a mass spectrometer, atoms interact with a magnetic field and separate according to their mass to charge ratio. As they separate according to this ratio, their percentage abundance and relative atomic masses can be calculated. Let’s use the following example to illustrate how the relative mass of an atom is calculated using carbon-12 as the standard.
Example
If a chemist measured a sample in a mass spectrometer and determined that the mass ratio of 28Si (silicon-28) to 12C (carbon-12) is: 2.33, calculate the mass of 28Si?
Solution
Since the mass of carbon-12 is is assigned a value of 12, then it follows that the mass of 28Si = mass ratio of (28Si/12C) multiplied by mass of 12C
Thus, mass of 28Si = 2.33 x 12 amu = 27.98 amu
To learn more about atomic mass and Avogadro’s number, click here.