If a substance is homogeneous, is it a pure substance?
Homogeneous means the same or uniform throughout. Therefore, a homogeneous substance can either be a mixture or a pure substance. When a homogeneous substance consists of the same type of molecule with fixed and uniform composition throughout, then the substance is a pure substance.
For instance, in pure water, no other component is present except water molecules (H2O). And these water molecules are made when fixed amounts of hydrogen and oxygen atoms react. Therefore, pure water is homogeneous and pure substance.
However, when a homogeneous substance consists of two or more different types of molecules uniformly intermingled with one another, then it’s called a homogeneous mixture. A mixture’s composition can vary, but a pure substance does not.
For instance, air is a homogeneous mixture of nitrogen, oxygen, water vapor, and other gases in trace amounts. The proportion of these gases in air can vary.
Similarly, salt solution is a homogeneous mixture of salt and water. Yet, the amounts of salt and water can vary from one salt solution to another.
Therefore, we are more correct when we say that a pure substance is homogeneous, but not fully correct when we say that a homogeneous substance is a pure substance.
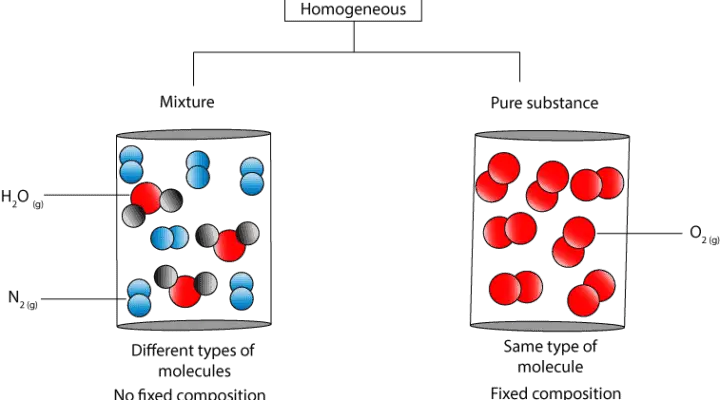
Let’s use the following particle models to further illustrate the difference.
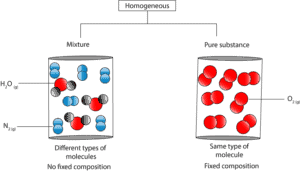
As you can see, in the homogeneous mixture the water and nitrogen molecules are uniformly distributed in the container. Yet, it’s composition is not fixed. Thus, if you sample from anywhere in the container, the proportion of nitrogen and water molecules can vary.
In contrast, in the pure substance, the oxygen molecules are uniformly distributed in the container, and its composition fixed. Thus, regardless of where you sample, the composition of oxygen molecules will always be the same.
To learn more about mixtures, click here, and about pure substance click here.