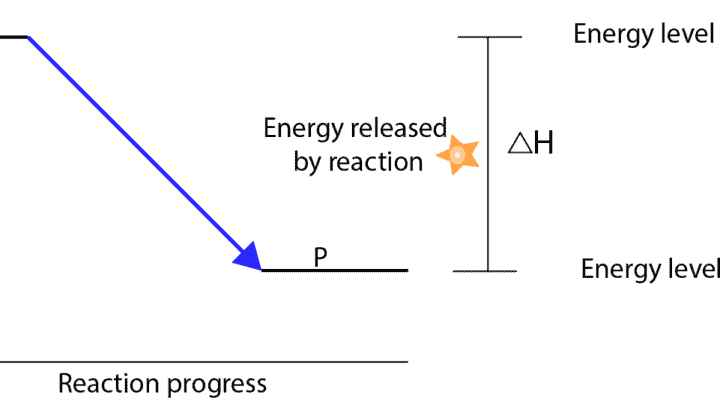
Why do some reactions release energy, while others absorb energy from the surroundings?
If reactant molecules in a particular reaction have more energy than the product molecules, then energy is released to the surroundings in the form of heat and light. And when this happens, the reaction is usually called an exothermic reaction.
On the other hand, if the product molecules have more energy than the reactants, then the reaction absorbs energy from the surroundings. And when this happens, the reaction is usually called an endothermic reaction.
We can graphically represent these energy changes when we plot energy on the y-axis and reaction time on the x-axis. When we plot one for an exothermic reaction, it will look like this:
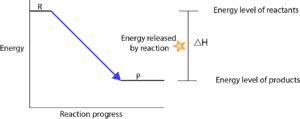
As you can see, the reactants (R) are at a higher energy level than the products (P). This means that the reactants have more energy than the products. Also, the reaction can occur without absorbing energy from the surroundings. For this reason, chemists usually say the path from reactants to products is “downhill” in energy.
When you subtract the energy of reactants (HInitial) from the energy of products (Hfinal), you will get what chemists call the Enthalpy change, which is usually represented by the symbol, ∆H (read as delta H). For exothermic reactions, ∆H is always less than zero (∆H<0).
Enthalpy change or heat of reaction is equal to the energy gained or lost only when a reaction is carried out at constant pressure.
Similarly, an energy profile diagram for an endothermic reaction is:
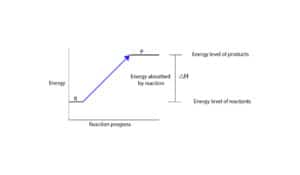
As you can see, endothermic reaction is the opposite of an exothermic reaction. The reactants are at a lower energy level than the products. Therefore, in endothermic reactions, the enthalpy change is always greater than zero (∆H>0). And for the reaction to occur, it must absorb energy from the surroundings. For this reason, chemists usually say the path from reactants to products is “uphill” in energy.
Can molecular structure be used to estimate enthalpy change (∆H)?
Yes, it can. Chemists understand that when the same type of bond is found in more than one molecule, about the same amount of energy is needed to break each one. This means that chemists can describe chemical bonds by bond energy. What is bond energy? Bond energy is simply the energy needed to break a bond.
To use bond energies to estimate ∆H, we must view chemical reactions as a process that breaks and makes chemical bonds. To break chemical bonds, the reacting molecules usually absorb energy. To make chemical bonds, the reacting molecules usually release energy. Therefore, the difference in energy between bond breaking and bond making determine whether energy is absorbed or released to the surroundings.
Once we digest the previous statement, then we can say that:
- when the energy absorbed in bond breaking is greater than the energy release in bond making, then the reaction is endothermic
- when the energy absorbed in bond breaking is less than the energy release in bond making, then the reaction is exothermic
Why does the energy absorbed or released vary when molecules react?
When reactant molecules have stronger bonds, more energy is absorbed from the surroundings to break them. When product molecules have weaker bonds than the reactant molecules, little energy is released to the surroundings when the product molecules form.
When reactant molecules have weaker bonds, little energy is absorbed from the surroundings to break them. When product molecules have stronger bonds than the reactant molecules, more energy is released to the surroundings when the product molecules form.
To learn why atoms bond, click here.