How do atoms form ions?
An ion is formed when an electron is removed or added to an atom. Pay attention to the word in bold: electron. Recall that an electron has a negative charge, while a proton has a positive charge. That means when an electron is removed from an atom, the number of electrons become fewer than the number of protons, and since protons carry a positive charge, the whole atom now carries a positive charge, and this positively charge atom is called a cation.
Likewise when an electron is added to an atom, the number of electrons become more than the number of protons, and since electrons carry a negative charge, the whole atom now carries a negative charge, and this negatively charged atom is called an anion. Now lets use the following examples to further explain how atoms for ions
How does sodium atom become positively charged?
In neutral sodium atom, you have 11 electrons and 11 protons. Here is model showing the number of each:

When neutral sodium loses an electron it becomes a sodium cation. It does because the number of protons becomes more than the number of electrons. Here is a model showing these numbers:

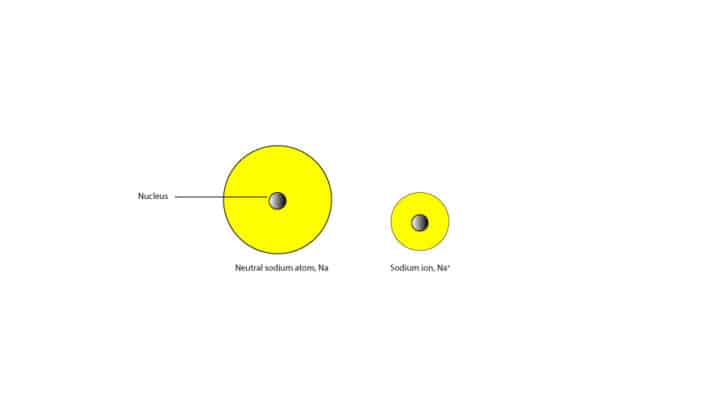
As a result of the electron loss, the many protons (11) strongly attract the fewer electrons (10) shrinking the size of the atom. For this reason, sodium cation is smaller than the neutral sodium atom. Here is a model showing their relative sizes.
Generally, cations are smaller than their neutral atoms.
How does chlorine atom become negatively charged?
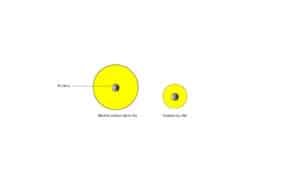
When neutral chlorine receives an electron it becomes a chloride ion. This chloride ion (anion) becomes larger than the neutral chlorine atom. This happens because the fewer protons in the nucleus of chlorine can’t strongly attract its many electrons. As a result, chloride ion becomes larger than its neutral atom. Here is a model showing the number of protons and electrons:

Here is a model showing their relative sizes.
Generally, anions are larger than their neutral atoms.
To learn why a cation is smaller than its neutral atom, or why an anion is larger than its neutral atom, click here
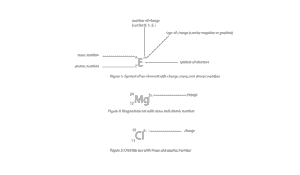
What symbols can we use to describe an ion?
Figure 1 is the general way of writing a symbol for an ion.
To learn how to calculate the number of electrons in an ion, click here.
To learn more about subatomic particles, click here.