What’s reaction rate and what factors can affect it?
How fast reactants react or how fast products appear in a chemical reaction is called reaction rate. Reaction rate is determined by energy changes when reactants are converted to products.
In general, before products can appear in a reaction, the reacting molecules must absorb energy to break old bonds so that their atoms can regroup to form new bonds: products. As a result, almost all chemical reactions require energy in order to proceed.
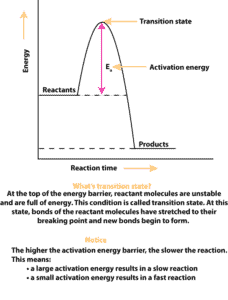
This energy requirement creates an energy barrier between the reactants and the products. This energy barrier determines how hard the reactant molecules must collide so that the reaction can go (break bonds). This energy barrier is usually called the ACTIVATION ENERGY (EA).
Activation energy is the minimum amount of energy the reactants must absorb to make the reaction go. Graphically, activation energy is the difference between the initial energy of the reactants and the energy from the top of the energy barrier. This difference is illustrated by the red arrow in the following model:
Since the rate at which products appear depends on the concentration of the reactants in moles per liter, we can formally define reaction rate as a quantity that’s proportional to the concentration of the reactants raised to certain powers.
For example, when reactants A and B react to make products C and D, we can write the reaction equation as:
aA + bB → cC + dD
From this equation, we can write the rate expression as: Rate ∞ [A]x[B]y
where;
[A] = concentration of reactant A in moles per liter
[B] = concentration of reactant B in moles per liter
x and y are called the orders of the reaction. Values of x and y can be whole, fractional, or zero.
If we remove the proportionality sign and introduce a proportional constant and an equal sign, we will get: Rate = K[A]x[B]y
where
k, the proportionality constant is called the rate constant. Its value depends on the reactions’ activation energy, shape and orientation of reacting molecules.
Can the values of x and y be equal to the coefficients of the reactants in the balanced equation?
Yes, they can, but not always. The actual values of x and y can only be determined by rate experiments called kinetics. These values, which are usually called reaction orders, tell us how reactant concentration affects reaction rate.
For instance, imagine the rate expression, Rate = K[A]1[B]2, where reactant A has an order of 1 and reactant B an order of 2. The order 1 usually means that when we double the concentration of A, while the concentration of B is held constant, the reaction rate doubles too. Similarly, the order 2 means that when the concentration of B is doubled, the rate quadruples. As a result, in rate experiments, we usually vary the concentration of one reactant while we measure the reaction rate. For instance, if after one such experiment, a chemist determine that a reactant concentration has no effect on the reaction rate, then the reaction order of such a reactant is zero.
How to measure reaction rate
Imagine that we put 1 M (1 mol/1L) of reactant A in a one-liter vessel. Another 1 M (1 mol/1L) of reactant B (gaseous molecules) in the same vessel. If we run the reaction and then we determine that after 1 minute, 0.1 M of the product (0.1 mol/1 liter) forms, then, we can calculate the reaction rate as:
Rate = Change in concentration of product divided by the Time in minutes.
Since no product was present at the start of the reaction, we can say that the initial concentration of the product is zero. Therefore, the change in product concentration will be the difference between the initial concentration and the concentration after the 1 minute. Thus, 0.1 M – 0.0M divided by 1 minute (0.1 M/1 minute) is equal to 0.1 M/minute. Here is a model showing the concentration of the reactants and product before and after 1 minute.
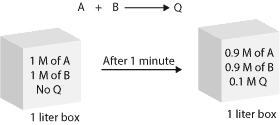
What factors can affect reaction rates?
Factors that can affect reaction rate include
- Temperature
Temperature is the average kinetic energy of a collection of molecules. This means that as temperature increase, the average speed and kinetic energy of the reacting molecules will increase. This increase in kinetic energy will cause many reacting molecules to collide more often. However, not all collisions can increase the reaction rate. Only molecules that collide with the right energy and orientation can overcome the energy barrier and increase the reaction rate.
- Concentration of reactants
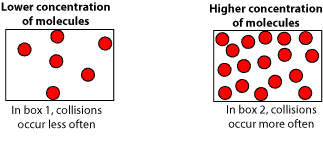
As the concentration of reactants increase, reactant molecules collide with other reactant molecules more often, increasing the reaction’s rate. Let’s use the following models to illustrate.
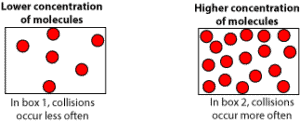
From the models, you can see that box2 has more molecules (higher concentration) than box1 (lower concentration). Therefore, the reaction rate will be faster in box2 than in box1.
- Catalyst
A reaction with a high activation energy results in a slower reaction. A slower reaction can be increased by increasing the reaction’s temperature. However, there are limits to how high you can increase the temperature. For example, some reactants are highly sensitive to temperature and will decompose if the temperature is increased too high or cause other unwanted reactions (side reactions) to occur.
So how do chemists get around the limitations of increasing the reactions temperature?
They use a catalyst. A catalyst is a substance when added to a reaction increases the reaction rate by lowering the activation energy but remains substantially unchanged after the reaction is complete. A catalyst lowers the activation energy by providing a new mechanism– an alternative path for reactants to combine to form products.
To learn about reaction rates and chemical equilibrium, click here.
Question
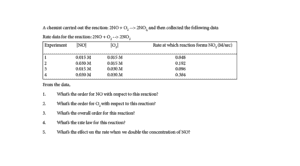
Use the data from the table below to determine the rate law for the reaction:
- 2NO + O2 → 2NO2