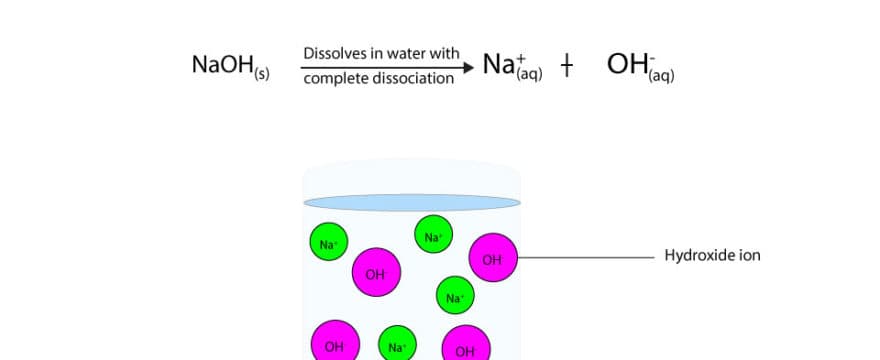
Sodium hydroxide (NaOH) is strong base because it fully dissociates in water to produce hydroxide ions. While ammonia (NH3) is weak base because it accepts protons from water to produce fewer hydroxide ions in solution. Because strong bases fully dissociate in water, they produce lots of hydroxide ions in solution, making the solution more basic. While weak bases produce fewer hydroxide ions, making the solution less basic.
If we write an equilibrium equation for sodium hydroxide, here is how it will appear.

From the equilibrium equation, you can see that a single arrow points to the right. This arrow conveys the message that strong bases fully dissociate in water and that the equilibrium for the dissociation lies largely to the right
Thus, the more hydroxide ions you have in solution, the larger the base dissociation constant (Kb) and the stronger the base.
Here is a molecular level diagram showing the complete dissociation of NaOH in water.
If we write the equilibrium equation for NH3, here is how it will appear:
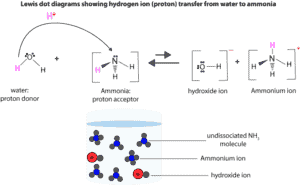
As you can see, ammonia does not dissociates, it only accepts a proton from water to generate the hydroxide ion. The shorter arrow pointing to the right shows that only few ammonia molecules accepts a proton from water to produce hydroxide and ammonium ions. However, these ions easily combine to get back ammonia and water. Meaning the equilibrium lies largely to the left, which is why we drew the reverse arrow longer than the forward arrow.
Generally, we can write the equilibrium equation for a weak base as:
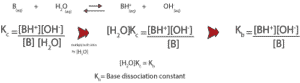
We get the base dissociating constant, Kb when we multiply the equilibrium constant, Kc by [H2O]. We did this because for dilute solution the concentration of water is relatively constant.
You will also notice in the molecular level diagram that only few NH3 molecules accept protons from water to generate hydroxide and ammonium ions.
If you want to learn more about strong acids and weak acids, click here, and about electrolytes click here.