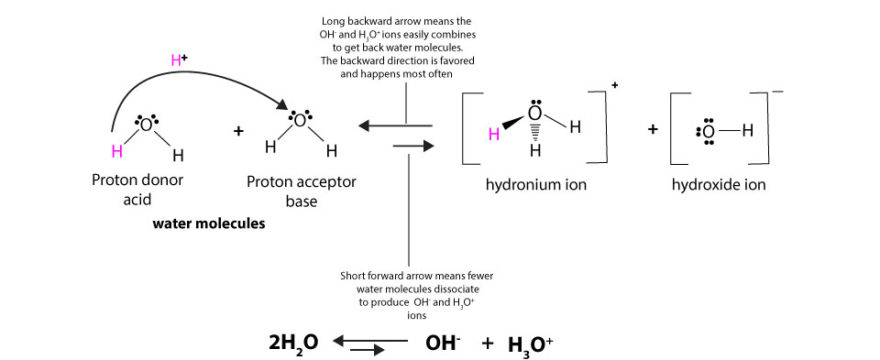
When is solution acidic or basic?
An aqueous solution is acidic when its hydronium ions (H3O+) are greater than its hydroxide ions (OH–).
An aqueous solution is basic when its hydroxide ions (OH–) are greater than its hydronium ions (H3O+).
Thus, we can say that
- acidic solutions have a greater concentration of hydronium ions than hydroxide ions. We can express this concentration as: [H3O+] > [OH–]
- Basic solutions have a greater concentration of hydroxide ions than hydronium ions.
We can express this concentration as: [OH–] > [H3O+]
- Neutral solutions have the same concentration of hydroxide and hydronium ions.
We can express this concentration as: [OH–] = [H3O+]
The square brackets represent concentration: [concentration]. And here concentration means the number of hydronium(H3O+) or hydroxide (OH–) ions in a liter of solution.