Why does matter exists in different phases?
Matter exists in different phases (solid, liquid and gas) because of two main reasons. These reasons are changes in
- Temperature (average speed of molecules), and
- Intermolecular forces (attractive forces between molecules)
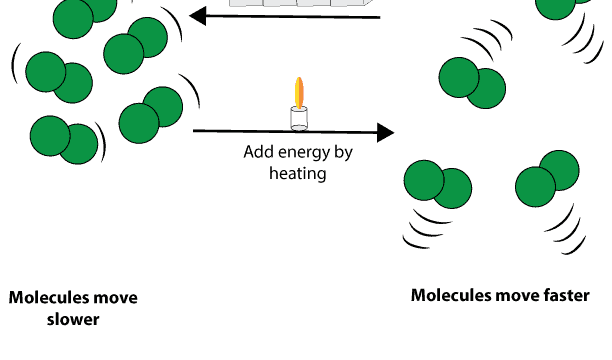
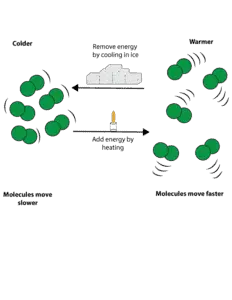
At the molecular level, changes in temperature is related to how fast molecules move. Therefore, if we add energy to any substance, for instance by heat, we also in turn increase the speed at which the molecules of the substance move.
As the speed of these molecules increase, their kinetic energy also grows until such a point that their kinetic energy is sufficient to overcome the force of attraction between them. Once these attractive forces are broken, molecules will move away from each other, causing the substance to change from solid to liquid or liquid to gas.
Conversely, if we remove energy from any substance by cooling, we also in turn slow down the speed of the substance molecules. As these molecules slow down, their kinetic energy also decreases until such a point that their attractive forces are sufficient to overcome their kinetic energy. Once the attractive forces become stronger than their kinetic energy, the molecules will move closer to each other, causing the substance to change from gas to liquid or liquid to solid.
Here is a model showing how energy can be added to substance by heating and removed by cooling.
To learn how water changes phases, click here.