How acidic or basic is an aqueous solution?
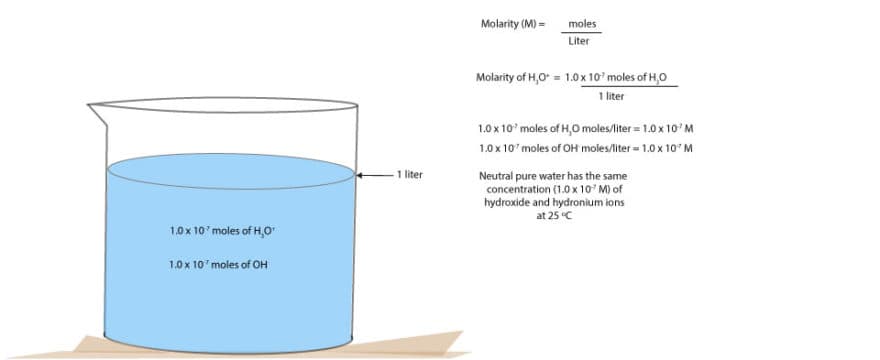
Since neutral water contains an equal number of hydroxide and hydronium ions, we can determine how acidic or basic a solution is by comparing its hydronium and hydroxide ions concentration to that of neutral water.

Thus,
- Acidic solutions have their hydronium ion concentration [H3O+] greater than 10-7
- Basic solutions have their hydroxide ion concentration [OH–] greater than 10-7
- Neutral solutions have the hydroxide ion concentration [OH–] equal to the hydronium ion concentration [H3O+]. Thus, [OH–]=[H3O+] = 10-14
Thus, the hydroxide and hydronium ions depend on each other. When the concentration of one increase, the concentration of the other must decrease. Except for neutral solution, in which both ions have exactly the same concentration:10-7M.
What’s the M in 10-7M? M is usually a short way of writing Molarity. Molarity is one way of expressing solution concentration. And it’s defined as the number of moles of ions or molecules in a liter of solution (moles per liter).
Example
You made hydrochloric acid solution by dissolving 1 mol of hydrogen chloride (HCl) gas in enough water to make a liter of solution. Calculate the solution’s hydroxide ion concentration.
Strategy
Since HCl is a strong acid, it will fully dissociate in water to produce 1 mol of hydronium (H3O+) ions.
Thus,
HCl(aq) + H2O → H3O+(aq) +Cl–(aq)
1 mol 1 mol 1 mol
Therefore, Molarity of H3O+ ions = moles/liter = 1 mol/1L = 1 M.
The 1 M H3O+ ions concentration is far greater than 10-7 M of H3O+ ions in neutral water. This shows that 1 M HCl solution is highly acidic.
To calculate the hydroxide ion concentration (OH–), you have to recall that:
Kw = [OH–] x [H3O+], however, you do know that Kw = 10-14
Therefore, we can write that: 10-14 = [OH–] x [1]
[OH–] = 10-14
Since the [OH–] concentration is smaller than the [H3O+], it agrees with the previous calculation that the solution is highly acidic. Clearly, when one of the ions concentration is known, you can always use the Kw expression to calculate the concentration of the other.