How can water make an aqueous solution acidic or basic?
Because of the ability of water to self-dissociate to generate little amounts of hydronium(H3O+) and hydroxide (OH–) ions, solutions prepared with water as solvent (aqueous solution) can become acidic or basic when an acid or base is added to water. Water self-dissociates when one water molecule donates a proton to another water molecule. The water molecule that donates the proton becomes the hydroxide ion (OH–), while the water molecule that accepts the proton becomes the hydronium ion(H3O+). Rarely will you find pure water without little amounts of these two ions in it. Therefore, we can write an equation for the dissociation of water as:
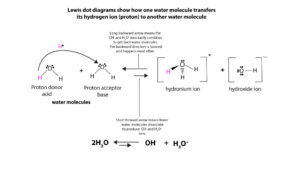
As you can see, because the equilibrium largely lies to the left, the longer arrow points to the left. Meaning only fewer water molecules dissociate to produce hydronium and hydroxide ions. Because only few water molecules dissociate, the concentration of the hydronium and hydroxide ions are significantly lower in water. Also notice that water is both the proton donor (acid) and proton acceptor (base). Because of this behavior, water dissociates to produce equal number of hydronium (H3O+) and hydroxide (OH–) ions. And when there are the same number of hydronium and hydroxide ions, the solution is called a neutral solution. Meaning the solution is neither acidic nor basic.
From the auto-dissociation of water,
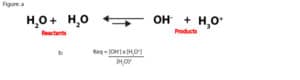
we can write its equilibrium constant expression as Keq = [H3O+] x [OH–]/ [H2O]2. This expression is the same as the one in figure b. The equilibrium constant is obtained when we divide the concentration of the products raised to their appropriate powers by the concentration of the reactants raised to their appropriate powers. Notice that, together, we have two moles of water on the reactants side. Because of this, the concentration of the reactants in the equilibrium expression is raised to the power, 2.