What’s Bohr’s atomic model?
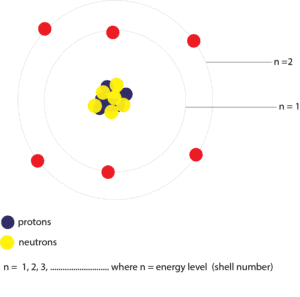
As you can see, in Bohr’s model the electrons now move in shells called energy levels. The shell closest to the nucleus is called the n = 1 principal energy level, and next, n = 2 principal energy level and so on. These energy levels have specific amounts of energy associated with them. So as electrons move in these energy levels they are also tied to this amount of energy. For an electron to move from one energy level to another, it must absorb the precise amount of energy that will move it to that energy level. Likewise, this same electron must lose the same amount of energy to move back to its original energy level. This back and forth movement of an electron between energy levels is called electron transitions.
What questions did critics raise about Bohr’s atomic model?
One of the questions critics raised was that Bohr assumed as if he knew the shells in which electrons orbit around the nucleus. This assumption did not agree with a law of physics called uncertainty principle. Another was that Bohr’s theory could not explain why scientists observed many spectral lines for elements with more electrons than hydrogen.
To answer these questions, scientists developed a theory called quantum theory or quantum mechanics. This theory takes into account the uncertainty principle.
What’s the uncertainty principle?
This principle states that “we always have a limit to how accurate we can measure an object, and this limit becomes more important when we try to measure tiny particles like an electron.” So why can’t we see an electron? We can’t see an electron because of at least two reasons:
- First, because the electron is really small, we need light of low energy so that when we shine light on the electron it wouldn’t dance around that much. If the electron dances around that much, by the time the light returns to our eyes, we won’t be able to see it.
- Second, because the electron is really small, we need to use light that has a wavelength shorter than the size of an electron so that we can focus the image clearly. Why is light of shorter wavelength necessary? It is an optical principle that for you to clearly see an object, the object must be larger than the wavelength of the light used to shine on it.
Now, only light in the form of gamma rays has wavelength shorter than the size of an electron. However, light in the form of gamma rays has so much energy that it will cause the electron to dance around. Because of this, it is practically impossible to see an electron. As a result, many scientists think that we may never be able to see an electron, and this conclusion is consistent with the uncertainty principle.
What’s the quantum theory of an atom?
The quantum theory takes into account the uncertainty principle and uses complex mathematical calculations to give us a blurry picture of regions around the nucleus where there is a higher chance that an electron may be there. These regions are usually called orbitals, and they describe the shape of the space in which electrons may exist. In fact, these orbitals give us a fuzzy theoretical picture of an electron. There are many of these orbitals, and here are a few of them:
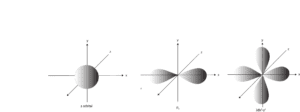
Now, when the 1s, 2s, and 2py orbitals are put together, you will see a picture like the one below:
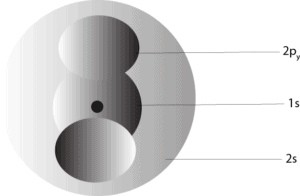
To read about how these orbitals are arranged in an atom, click here.
To learn more about Bohr and the quantum mechanical model of an atom, click here.