What’s Dalton’s Atomic theory?
Dalton’s atomic theory consists of a series of suggestions called postulates. These postulates are:
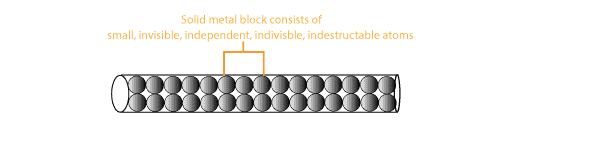
- All elements consist of small independent, indestructible and invisible particles called atoms.
He is a model to help picture the previous statement
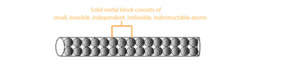
- All atoms of the same element have equal mass. While atoms of different elements have different mass, and the mass of these atoms remain the same after a physical or chemical change.
Note that today we know atoms of the same element can have different masses. Atoms of the same element with different masses are usually called isotopes.
Here is a model showing atoms of different elements with different masses
- Few atoms of different elements usually bond to form a small particle of a compound. These small particles are identical in number and the types of atoms they contain, and a larger mass of a substance is simply an accumulation of these small particles.
Are all Daltons’ ideas about the atom true today?
No! At present, scientists can split an atom by bombarding it with neutrons. Also, we now know that different versions of the same element (isotopes) can exist. As a result, atoms of the same element can have different masses.
What model did Dalton use to depict an Atom?
Since atoms are invisible, Dalton had to generate an atomic model to help explain his ideas about the atomic theory. The model he generated was called the “hard-sphere bullet model” which is often depicted on a paper as a circle. Here is the model:
What questions did critics raise about Dalton’s atomic theory?
Some scientist at that time suspected that an atom may consists of other smaller particles. They had seen that certain materials easily picked up charge when they are rubbed against other materials. For example, some had seen that when they rubbed a comb with a piece of wool, it easily picked up small pieces of papers.
To determine whether an atom consists of other tiny particles, one scientist called J. J. Thomson carried out a now famous experiment: cathode ray tube.