What’s Rutherford atomic model?
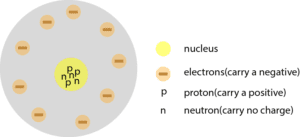
Rutherford’s atomic model consists of a nucleus. This nucleus contains positively charged particles (pluses) called protons. Years later, a second particle, called the neutron, was also discovered in the nucleus. Together, the atom contains three particles:
- protons (particles with positive charge) located in the nucleus
- neutrons (particles with no charge) located in the nucleus
- electrons (particles with negative charge) located in the electron cloud
The mass of a proton and a neutron are about equal and larger than the mass of an electron. The mass of an electron is so small that it can be ignored.
Now, here is a redrawn model with the neutrons included
What experiment led Rutherford to his atomic model?
To test the stability of Thomson’s atomic model, Rutherford carried out the now famous gold-foil experiment.
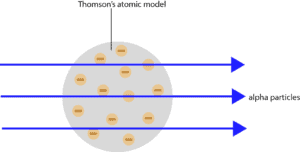
What were Rutherford’s predictions before conducting the gold-foil experiment?
Rutherford had predicted that the alpha particles will pass through the atom undeflected. Here is a diagram showing Rutherford’s prediction:
What did Rutherford fire at the gold-foil?
During the experiment, Rutherford fired positively charged alpha particles at the gold foil. These alpha particles are helium ions.
What were the results of the gold-foil experiment?
Here is a diagram showing the results of the experiment.
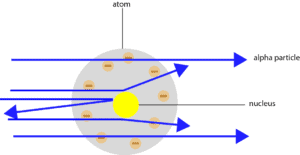
From the results, Rutherford concluded that the:
- atom consists of mostly empty space. How did he come to this conclusion? He observed that most alpha particles passed through the gold foil undeflected.
- atom consists of a dense center, called nucleus. How did he come to this conclusion. He observed that some alpha particles bounced off from the nucleus, while others were deflected through large and small angles.
- atom consists of a positively charge nucleus. How did he come to this conclusion? He observed that no alpha particle was sucked into the nucleus. Notice that alpha particles carry a positive charge, and like charges repel, while unlike charges attract.
What’s Rutherford atomic model (nuclear model)?
Based on the results, Rutherford proposed a new atomic model,which is shown below.
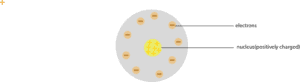
The nucleus contains positively charged particles (pluses) called protons. Years later, a second particle, called the neutron, was also discovered in the nucleus. Together, the atom contains three particles:
- protons (particles with positive charge) located in the nucleus
- neutrons (particles with no charge) located in the nucleus
- electrons (particles with negative charge) located in the electron cloud
The mass of a proton and a neutron are about equal and larger than the mass of an electron. The mass of an electron is so small that it can be ignored.
Now, here is a redrawn model with the neutrons included

What questions did critics raise about Rutherford’s atomic model?
Although Rutherford’s atomic model was an improvement of Thomson’s plum pudding model, many critics raised questions about how the electrons moved around the nucleus.
One was that
- since the electrons carry a negative charge and protons a positive charge, why aren’t the electrons sucked into the nucleus by the protons?
Another was that
- how did the electrons move around the nucleus, and not lose energy and collapse into the nucleus.
To answer these questions, Bohr proposed another atomic model.