What’s Thomson’s atomic theory?
To determine whether atoms really consist of other particles, a scientist called J. J. Thomson carried out the now famous cathode-ray tube experiment from which he concluded that the:
- cathode-rays consist of negatively charge particles. How did he come to this conclusion? He had observed that the cathode-rays travelled from the cathode (negatively charged) to the anode (positively charged).
- negatively charged particles were part of the atom. How did he come to this conclusion? He had observed that the particles were lighter than the lightest element: Hydrogen.
- negatively charge particles can be found in all atoms. How did he come to this conclusion? He had observed that no matter the metals he used as electrodes, the cathode-rays properties never changed.
From these conclusions, Thomson modified Dalton’s atomic theory and proposed a new atomic model popularly known as “plum pudding”, and this model appear in Figure 1.
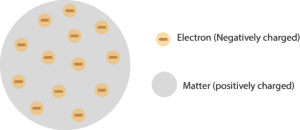
Notice, the electrons are the negatively charged particles, and these particles are embedded in the positively charged sphere (grey matter).
What questions did critics ask about his atomic model?
Some scientists questioned the stability of an atom based on his model. In other words, many scientists including his student, Rutherford, had no confidence in his model.
To test the stability of Thomson’s atomic model, Rutherford carried out the now famous gold-foil experiment.