How do you calculate the pH of strong acid?
To calculate the pH of strong acid, you must recall that strong acids completely dissociate in solution. What this means is that the ions in strong acids are separated from each other in water to produce positive ions called hydronium ions and negative ions (anion). Once we know this, we can write the following equation for any strong acid dissociating in water:

From the equation above, you can see only a single arrow pointing in the forward direction. This usually means that strong acids dissociate 100 % to produce the products (hydronium ion and anion). A fancy way of saying this is that the dissociation goes to completion.
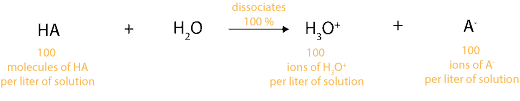
As an analogy, this means if we have 100 molecules of strong acid (HA) in a liter of solution, it will dissociate to produce 100 positive ions (hydronium ions) in a liter of solution and 100 negative ions (A–) in a liter of solution. Let’s illustrate this dissociation in the following equation:

Once we understand that strong acid dissociates 100 %, calculating its pH becomes less tedious. Thus, we need to take the negative log of the hydronium ion concentration to get its pH. That is: pH = -log [H3O+]
Now, let’s apply this understanding to calculate the pH of strong acid (HCl) in the following example.
Example
Calculate the pH of 0.02 M HCl (hydrochloric acid).
Strategy
Recall that M means concentration in Molarity, which is the same as moles per liter of solution.
Answer
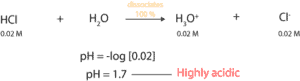
From the equation, since HCl is a strong acid, it dissociates 100%. Therefore, 0.02 M HCl will dissociate to produce 0.02 M H3O+. And since pH is defined as the negative log to the base 10 of hydronium ion concentration, we simply must take the negative log of 0.02 M. If we do, the pH of 0.02 M HCl solution is 1.7. Since 1.7 is far below pH 7, the solution is highly acidic. To learn about the pH scale, click here.