What’s Brønsted-Lowry’s concept of an acid and what’s Brønsted-Lowry’s concept of a base?
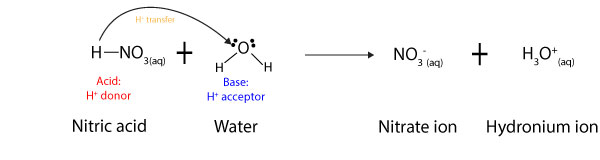
To Brønsted and Lowry, acids are chemicals that donate a hydrogen ion, while bases are chemicals that accept it. However, in Brønsted and Lowry sense, a chemical can only act as a base if and only if it has unshared electrons to share with the hydrogen ion. Let’s use the following equation to explain this further:
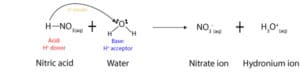
As you can see from the equation above, nitric acid donates a proton, while water accepts it (see arrow). Once it donates the proton to water, the nitric acid turns into a nitrate ion, while the water turns into a hydronium ion. As a result of this behavior, Brønsted and Lowry defined an acid as a chemical that donates a proton, while a base is a chemical that accepts a proton. For this reason, in acid-base reactions, acids are usually hydrogen ion (H+) donors, while bases are hydrogen ion acceptors.
Why did Brønsted and Lowry modify Arrhenius definition of a base?
They modified Arrhenius definition of a base because not all bases dissociate in water to produce hydroxide ions (OH–). To Arrhenius, a base is a chemical that dissociates in water to produce hydroxide ions (OH–), while an acid is a chemical that dissociates to produce hydrogen ions. Yet, ammonia (NH3), a base, does not dissociate in water to produce hydroxide ions. Rather, it accepts a hydrogen ion (proton) from water to generate the hydroxide ions. Let’s explain this further with the following models:
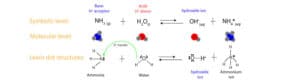
As you can see, the nitrogen in the ammonia molecule uses its unshared pair of electrons to accept a hydrogen ion (proton) from water. See the arrow in the Lewis dot structures above. Once an ammonia molecule accepts a proton from water, the ammonia molecule turns into an ammonium ion, while the water molecule turns into a hydroxide ion.
As a result, in Brønsted and Lowry sense, is more fitting to define a base as a proton acceptor.
Now, if we combine Arrhenius concept of acids and bases and Brønsted-Lowry concept of acids and bases in a diagram, we will get something like this:

As you can see, Arrhenius was the first to describe at the molecular level that:
- acids display acidic properties because of the presence of hydrogen ions in acidic solutions,
while
- bases display basic properties because of the presence of hydroxide ions in basic solutions
Brønsted and Lowry, however, came to enhance Arrhenius definition to include chemicals such as ammonia.
You may also have noticed in the examples above that water can act both as Brønsted-Lowry acid and Brønsted-Lowry base. Because of this behavior, water can display both acidic and basic properties. That is, depending on conditions, water can either donate or accept a proton. As a result of this behavior, water is usually called an amphoteric substance.