What is the concept of “conjugate” in acid-base chemistry?
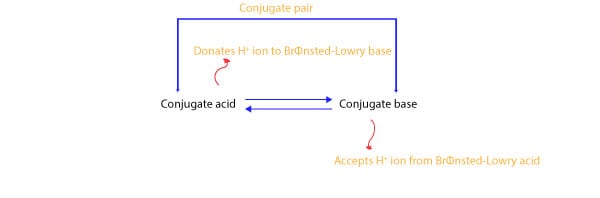
A conjugate means a “mate.” If we translate this meaning to acid-base chemistry, then we can say that every acid is tied to its mate called “conjugate base,” and together, they are called a “conjugate acid-base pair.” Similarly, every base is tied to its mate called “conjugate acid,” and together, they are called a “conjugate acid-base pair.” Therefore, every Brønsted-Lowry acid has its conjugate base. And every Brønsted-Lowry base has its conjugate acid. Let’s further explain this using the following model:
From the model, you can see that a conjugate acid is related to its conjugate base by the loss of a hydrogen ion, while the conjugate base is related to its conjugate acid by the gain of a hydrogen ion. That is a conjugate acid and its conjugate base are related by the loss and gain of a hydrogen ion. For instance, when a conjugate acid donates (loses) a hydrogen ion, it turns into its conjugate base. And when a conjugate base accepts a hydrogen ion, it turns into its conjugate acid.
Chemists sometimes call a hydrogen ion a proton. This is because a hydrogen atom, which has only one electron and one proton, becomes a positive hydrogen ion only when it loses its single electron. And when it does, it becomes a hydrogen ion with only one proton. For this reason, a hydrogen ion is the same as a proton.
Now let’s chemically illustrate conjugate acid-base pairs using the following example:

In the forward reaction (shown by the forward arrow in the equation above), you will notice that acetic acid, CH3COOH, a Brønsted-Lowry acid, donates a proton to water (H2O), and then turns into an acetate ion (CH3COO–), while water, a Brønsted-Lowry base, accepts it and turns into a hydronium ion (H3O+). Since the acetic acid (CH3COOH), and the acetate ion, CH3COO–, differ by only a proton (H+), we can say that the acetic acid (CH3COOH) and the acetate ion (CH3COO–) are conjugate acid-base pairs, where CH3COOH is the acid, and CH3COO–the base.
Similarly, in the reverse reaction (shown by the backward arrow in the equation above), the hydronium ion (H3O+), a Brønsted-Lowry acid, donates a proton to the acetate ion and then turns into water (H2O), while the acetate ion, a Brønsted-Lowry base, accepts the proton and turns into acetic acid. Since water (H2O) and hydronium ion (H3O+) differ by only a single proton, we can say that H2O and H3O+ are conjugate acid-base pairs, where H2O is the base and H3O+ the acid.
Notice that one member of the conjugate acid-base pair will always be a reactant, while the other a product. That is one member of the conjugate acid-base pair will always be on the left side of the chemical equation, while the other will be on the right side of it (see chemical equation above).